- Joined
- Mar 6, 2013
- Messages
- 1,537
- Reaction score
- 2,154
Hey guys. So I got a question wrong on bootcamp and now that I know it is wrong, I DO remember that molality is temperature independent. However, I am having a hard time understanding why.
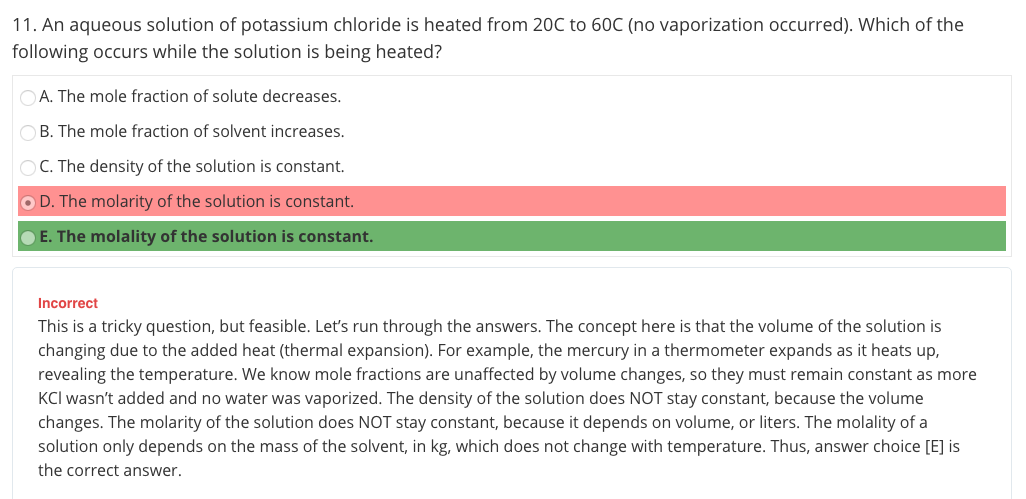
So it says that there is no vaporization change, so in my mind I think that the mass is constant, but why wouldnt the volume be constant? I imagine a pot of water that is being heated with an open top. If heat is added but there is no vaporization, how could there be a volume change if no liquid is evaporating?
I know that answer A and B is obviously incorrect. Density is mass / volume, and molarity is mol solute / L of solution. Molality is mol solute over kg solvent. Writing this out, I can see, from my old point of view, that C and D would be correct. However, knowing that molality is temperature independent, I still fail to see how there is a change in Liters, for molarity to no longer be constant (because no water evaporated)
After thinking about it, if you heat say 1 liter of water in a pot without vaporization, would the volume increase just because heat is added and the molecules expand from each other? In that case, would you still have 1 L of water? Maybe there would be 1.1 . Where is this mysterious water coming from?! Omg my brain right now!
PS: Sorry for the title typo.

So it says that there is no vaporization change, so in my mind I think that the mass is constant, but why wouldnt the volume be constant? I imagine a pot of water that is being heated with an open top. If heat is added but there is no vaporization, how could there be a volume change if no liquid is evaporating?
I know that answer A and B is obviously incorrect. Density is mass / volume, and molarity is mol solute / L of solution. Molality is mol solute over kg solvent. Writing this out, I can see, from my old point of view, that C and D would be correct. However, knowing that molality is temperature independent, I still fail to see how there is a change in Liters, for molarity to no longer be constant (because no water evaporated)
After thinking about it, if you heat say 1 liter of water in a pot without vaporization, would the volume increase just because heat is added and the molecules expand from each other? In that case, would you still have 1 L of water? Maybe there would be 1.1 . Where is this mysterious water coming from?! Omg my brain right now!
PS: Sorry for the title typo.
Last edited:
