- Joined
- May 5, 2008
- Messages
- 1,094
- Reaction score
- 4
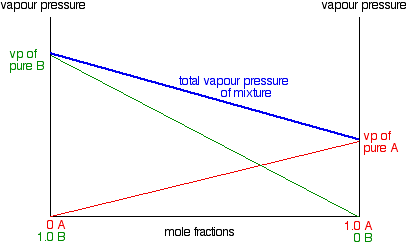
When you graph the total vapor pressures of a mixture (two volatile solvents), does the line have to have a negative slope or can it be positive? Check out the picture below:
[FONT=Helvetica, Arial]
 .
.
[FONT=Helvetica, Arial]

