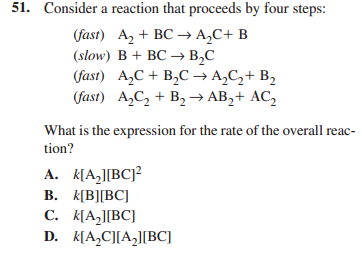
Answer: A
My question: Why isn't B the answer?

Why can't you use Hess's law to get the net reaction?
hess's law is relevant to thermodynamics and adding together delta H, delta S or delta G values.
i don't think it's applicable to kinetics.
No. You can't have an intermediate occur in your rate law. A2C is an intermediate. The right answer is A, which is in terms of only reactants and takes into account the double dependence on the concentration of BC.
Oh man, I must be having a day. You're all right that the answer to that one is D.
I'm going to go crawl into a corner now.
