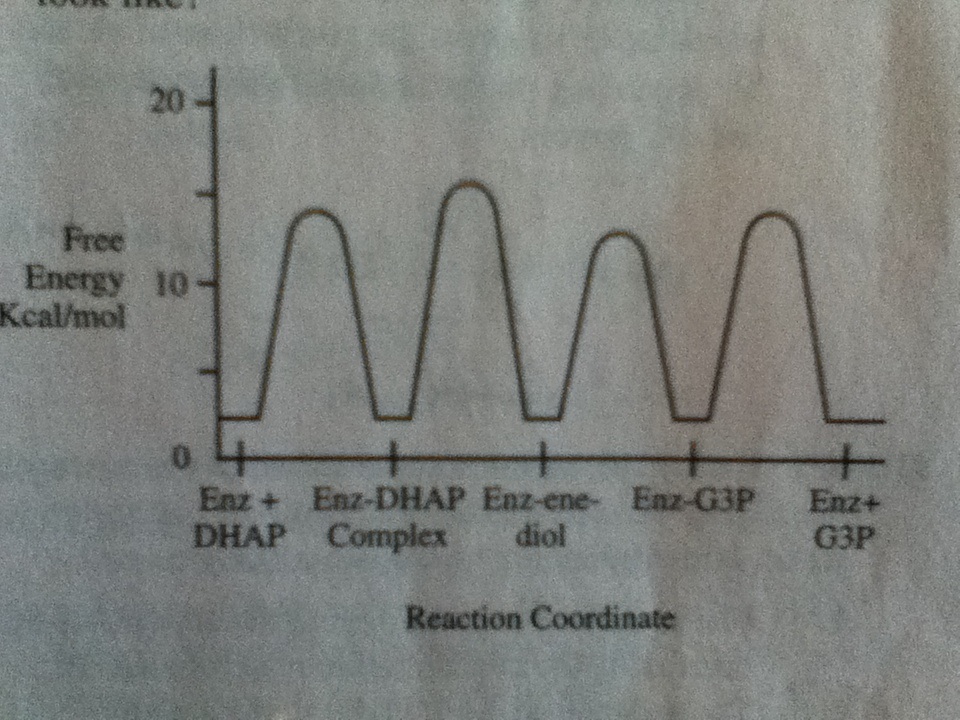
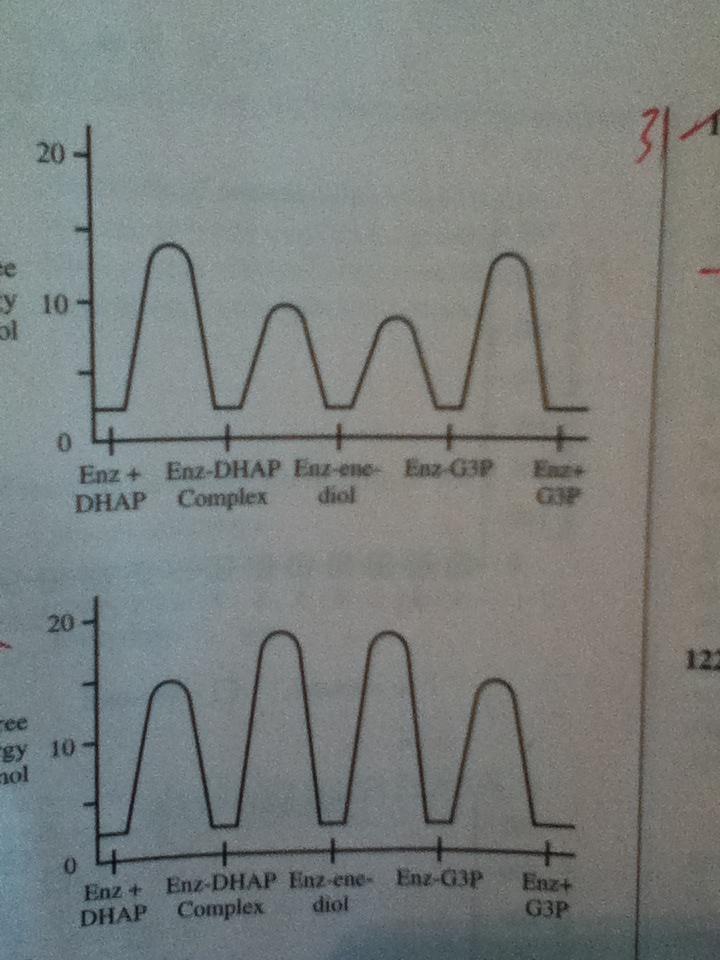
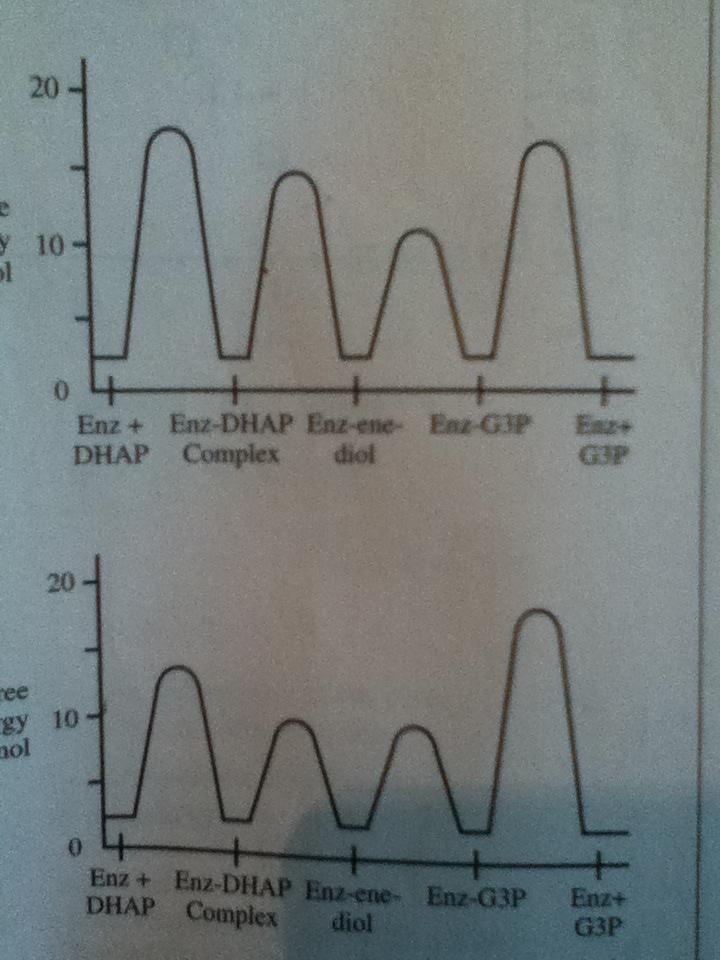
Way 1: Not sure if this is right, but it lead me to the right answer. You have a kinetic product and a thermodynamic product, right? And the kinetic product is faster and has a lower energy transition state, while the thermodynamic product is slower and higher energy. If the mutated enzyme makes the reaction proceed slower than the wild-type enzyme, then the thermodynamic product will probably be favored since it is more stable and a slower reaction means more time for the thermodynamic product to be formed (rather than the kinetic product continuously being formed). Thus, you would expect all of the free energy values to be higher.
Way 2: The shortcut way to this question though is to realize that (since they didn't give you any additional information) the change in free energy should be consistent for all E-S complexes in the graph. In other words, if one increases, they should all increase and if one decreases they should all decrease. Only graph B shows a consistent change so it must be the correct answer.
tl&dr:
Way 1: Slower rate leads to thermodynamic product which has higher energy. Graph B shows an increase in energy.
Way 2: Change in enzyme will be consistent for all the E-S complexes shown, only Graph B shows consistency across all 4 E-S complexes.