- Joined
- Jul 7, 2013
- Messages
- 617
- Reaction score
- 162
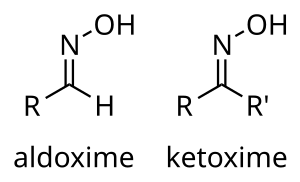
Is this true? Let me know if I"m the only person who's learned this "rule."

If this is true then why is the chlorine in chlorate anion sp3 hybridized?


If this is true then why is the chlorine in chlorate anion sp3 hybridized?

Last edited: