Hey!
I understand why B is the correct answer, however, I am confused why C would increase decrease blood acidity. If bicarbonate is converted into CO2, wouldn't blood acidity increase? Thanks!
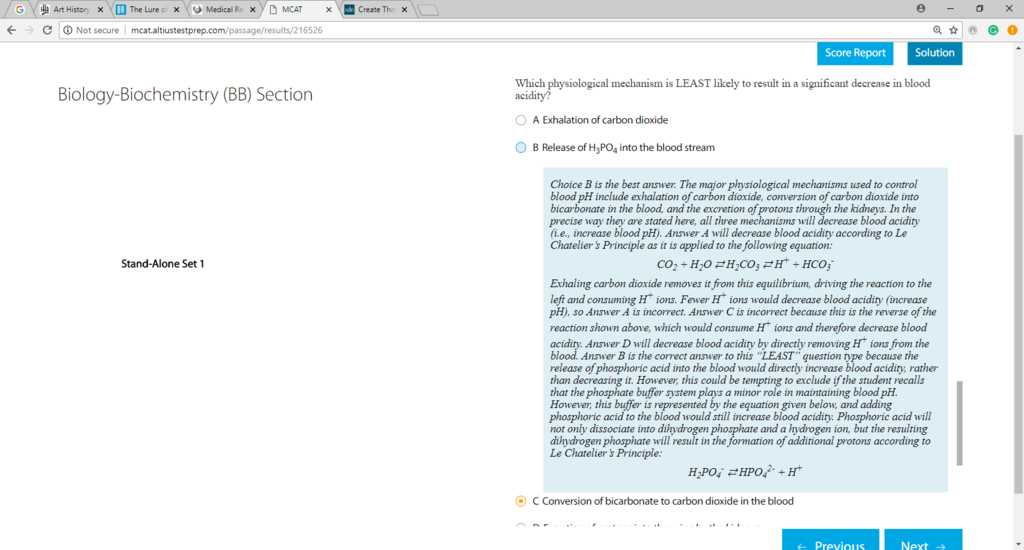
I understand why B is the correct answer, however, I am confused why C would increase decrease blood acidity. If bicarbonate is converted into CO2, wouldn't blood acidity increase? Thanks!
Last edited:
