- Joined
- Aug 20, 2007
- Messages
- 206
- Reaction score
- 0
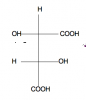
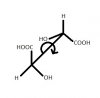
Does anyone know how to figure out the stereochemistry of this molecule? If you can please explain how you came up with answer. I have the answer but don't understand how the book came up with it. I'll share after a few posts.
This will prevent ppl. from trying to justify the answer.
This will prevent ppl. from trying to justify the answer.