loveapplejuice789
New Member
- Joined
- Jan 15, 2020
- Messages
- 2
- Reaction score
- 0
For question 4, A is the correct answer because "a less stable reactant yields a greater amount of heat upon reaction, so the pi-bond must be weakest in an unsubstituted alkene (making it the least stable of the alkenes)".
Applying the same logic, I thought 7 would be D because unsubstituted carbon would be least stable (weakest), but the solution manual said B. Can anyone explain this to me? Thank you!

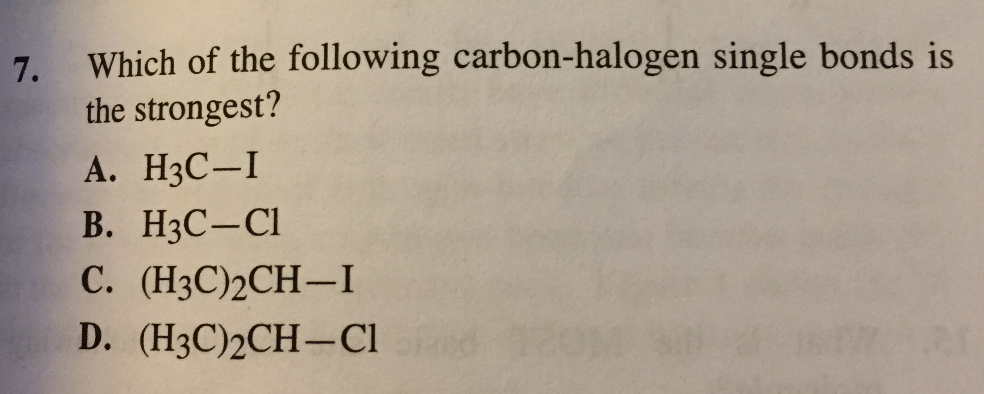
Applying the same logic, I thought 7 would be D because unsubstituted carbon would be least stable (weakest), but the solution manual said B. Can anyone explain this to me? Thank you!
