WTF at this entire chapter.
Given that S2O2 in a 0.5L flask is consumed at a rate of 0.008 moles per second, what is the formation rate of S4O6?
2 S2O3 + I2 -> S4O6 + 2I
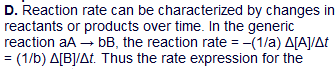
So the rate here is (1/1)S4O6 = -(1/2)S2O3
Nope. What am I doing wrong?
Given that S2O2 in a 0.5L flask is consumed at a rate of 0.008 moles per second, what is the formation rate of S4O6?
2 S2O3 + I2 -> S4O6 + 2I
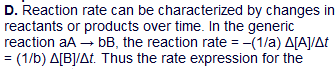
So the rate here is (1/1)S4O6 = -(1/2)S2O3
Nope. What am I doing wrong?
