- Joined
- May 10, 2015
- Messages
- 45
- Reaction score
- 26
Hi Folks,
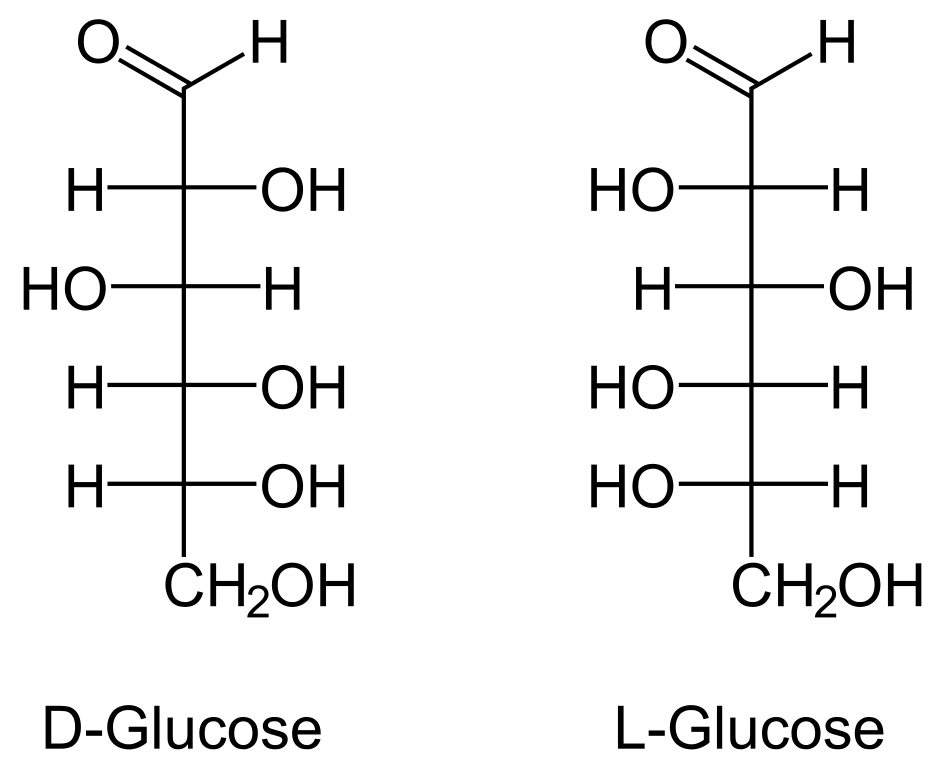
-I am not sure if any of you folks have bought the new TBR books. I had a quick question on #16 in Section VII of the Orgo Books under the Carbohydrates Review Questions. In this question, they ask you which structure represents the C6 deoxy form of L-mannose.
-I understand the logic which states that mannose is a C2 epimer of d-glucopyranose. I also understand that d-glucopryanose has all of its bulky groups in the equatorial position. This means that d-mannose would have all of its bulky groups in the equatorial position other than C2 where the hydroxyl group would be in the axial position.
TBR, however, asks for the chair confirmation of L-mannose and I wanted to confirm that L-mannose would essentially have all substituents in opposite config relative to D-mannose. If so, this would invalidate their answer.
Thanks,
plukfelder2017
@BerkReviewTeach (if you could provide some guidance I would appreciate it as well.)
***PS: your new books rock.
-I am not sure if any of you folks have bought the new TBR books. I had a quick question on #16 in Section VII of the Orgo Books under the Carbohydrates Review Questions. In this question, they ask you which structure represents the C6 deoxy form of L-mannose.
-I understand the logic which states that mannose is a C2 epimer of d-glucopyranose. I also understand that d-glucopryanose has all of its bulky groups in the equatorial position. This means that d-mannose would have all of its bulky groups in the equatorial position other than C2 where the hydroxyl group would be in the axial position.
TBR, however, asks for the chair confirmation of L-mannose and I wanted to confirm that L-mannose would essentially have all substituents in opposite config relative to D-mannose. If so, this would invalidate their answer.
Thanks,
plukfelder2017
@BerkReviewTeach (if you could provide some guidance I would appreciate it as well.)
***PS: your new books rock.
Last edited: