- Joined
- May 21, 2014
- Messages
- 38
- Reaction score
- 22
I agree with D being the answer to this question....
But I disagree with a part of the explanation:
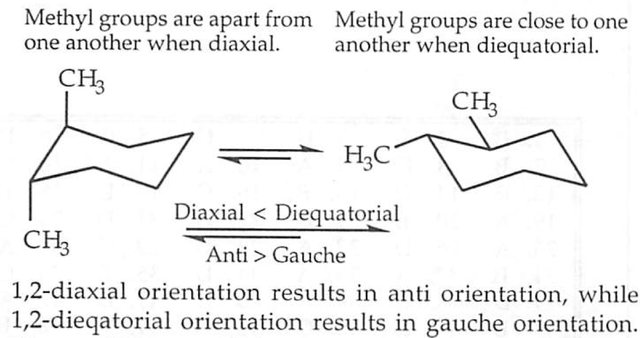
My qualms pertain to that last sentence. I recognize that in the 1,2-diaxial orientation, the methyl substituents are anti to each other. However, in this orientation, there are also two gauche interactions between the methyl substituents and the ring bonds (the CH2's). In the 1,2-diequatorial orientation, there is only one gauche interaction between the methyl substituents, and their orientations relative to the ring bonds are anti (2 anti, 1 gauche).
So the book is saying the diequatorial orientation is better for the reason of there being no axial steric hindrance albeit the gauche interaction, but the diequatorial orientation has more favorable anti interactions and fewer gauche than the diaxial orientation does.
I've drawn a picture to explain what I mean (I don't account for the two ring CH2's as gauche since their orientation is inherent to both conformers.):
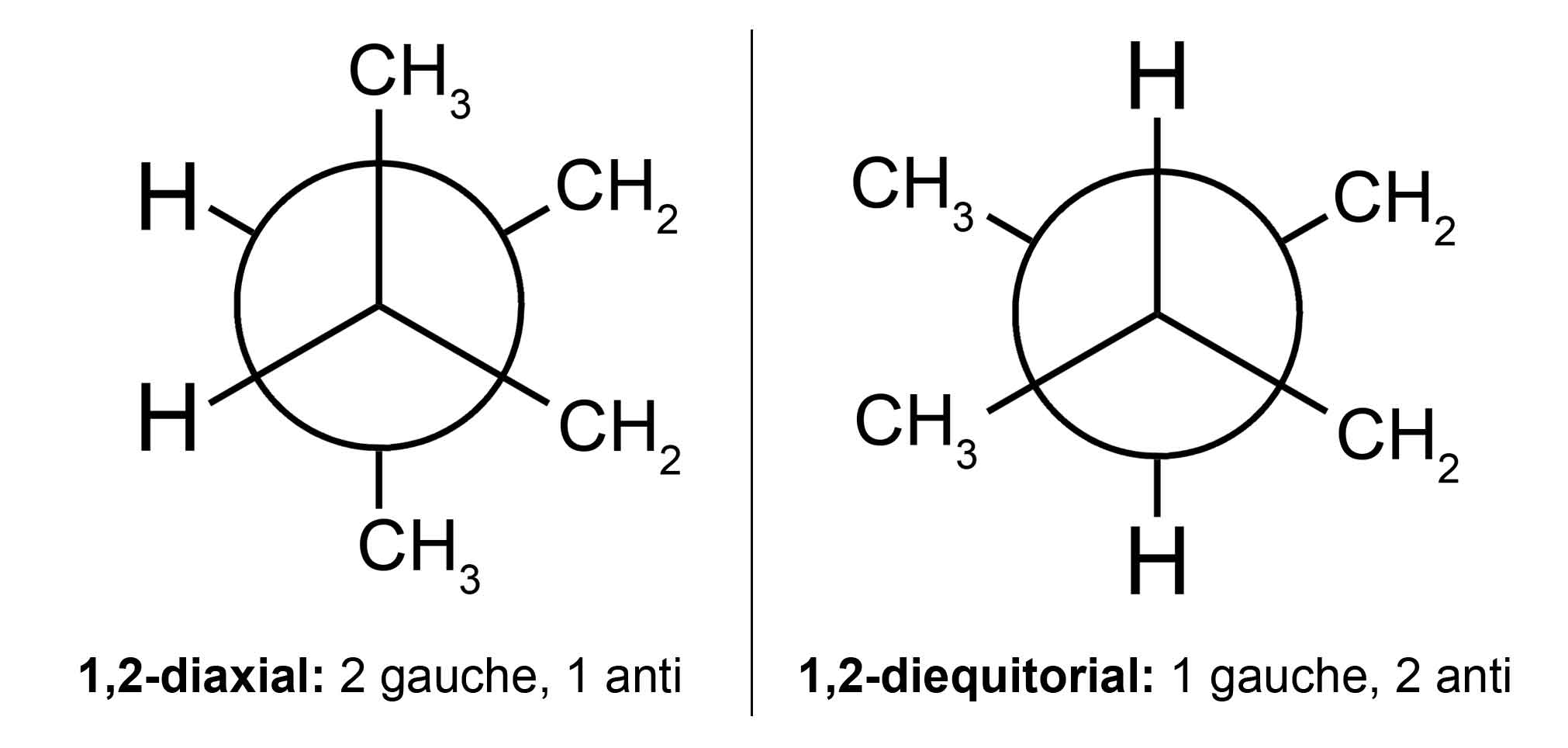
(http://imgur.com/sVHciNV)
What is the value of Keq(1,4)for the conversion of trans-1,4-dimethylcyclohexane from its conformation to its diequatorial conformation?
A . 0.0029
B. 2.16
C. 4.31
D. 345
A . 0.0029
B. 2.16
C. 4.31
D. 345
But I disagree with a part of the explanation:
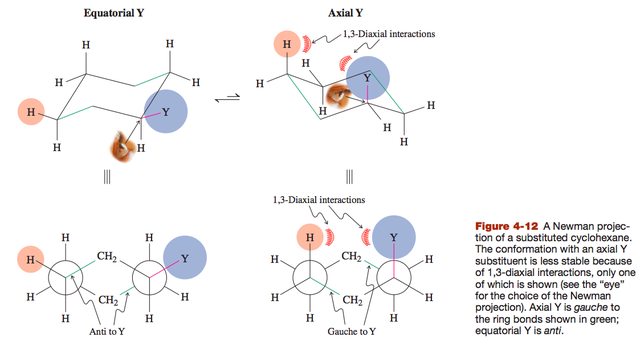
The diequatorial orientation is better than the diaxial orientation, because with diaxial there are eclipsed interactions with the axial hydrogens. However, the anti orientation of the methyl groups is better than the gauche orientation.
My qualms pertain to that last sentence. I recognize that in the 1,2-diaxial orientation, the methyl substituents are anti to each other. However, in this orientation, there are also two gauche interactions between the methyl substituents and the ring bonds (the CH2's). In the 1,2-diequatorial orientation, there is only one gauche interaction between the methyl substituents, and their orientations relative to the ring bonds are anti (2 anti, 1 gauche).
So the book is saying the diequatorial orientation is better for the reason of there being no axial steric hindrance albeit the gauche interaction, but the diequatorial orientation has more favorable anti interactions and fewer gauche than the diaxial orientation does.
I've drawn a picture to explain what I mean (I don't account for the two ring CH2's as gauche since their orientation is inherent to both conformers.):

(http://imgur.com/sVHciNV)
Last edited: