23. Which of the following statements is NOT true?
A. It is possible to sublime the solidA form of Material C at temperatures below the first triple point by changing the pressure.
B. It is possible for gas of Material C to undergo deposition to solids at temperatures between the first and second triple points.
C. Both Material T and Material C have two triple points each.
D. At standard temperature and pressure (0"C and 1.00 atm.), Material T exists as solidA-
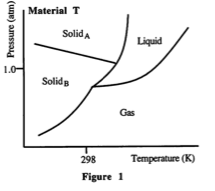
I picked C, because I looked at Material T and near the SolidA I see the solid and liquid intersecting, but didn't see how it was a triple point. Is it a triple point because Solid A, Solid B and Liquid are intersecting?
A. It is possible to sublime the solidA form of Material C at temperatures below the first triple point by changing the pressure.
B. It is possible for gas of Material C to undergo deposition to solids at temperatures between the first and second triple points.
C. Both Material T and Material C have two triple points each.
D. At standard temperature and pressure (0"C and 1.00 atm.), Material T exists as solidA-
I picked C, because I looked at Material T and near the SolidA I see the solid and liquid intersecting, but didn't see how it was a triple point. Is it a triple point because Solid A, Solid B and Liquid are intersecting?
