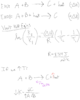6
663697
For endo/exothermic reactions, why does a change in temperature affect the Keq of the reaction? The sources that I've found refer to heat as a reactant/product, and the reaction shifts accordingly when adding/removing heat from the system. But why does this shift also change Keq, if changes in concentration/pressure also cause shifts but maintain the same Keq?