- Joined
- Jul 25, 2008
- Messages
- 228
- Reaction score
- 0
I don't get why is
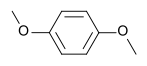 more soluble in pentane than
more soluble in pentane than
 I understand that its because ether is nonpolar, but 1,4 Diaminobenzene looks non-polar to me also, since it's symmetrical? I'm missing something here...
I understand that its because ether is nonpolar, but 1,4 Diaminobenzene looks non-polar to me also, since it's symmetrical? I'm missing something here...


