- Joined
- Feb 13, 2014
- Messages
- 507
- Reaction score
- 322
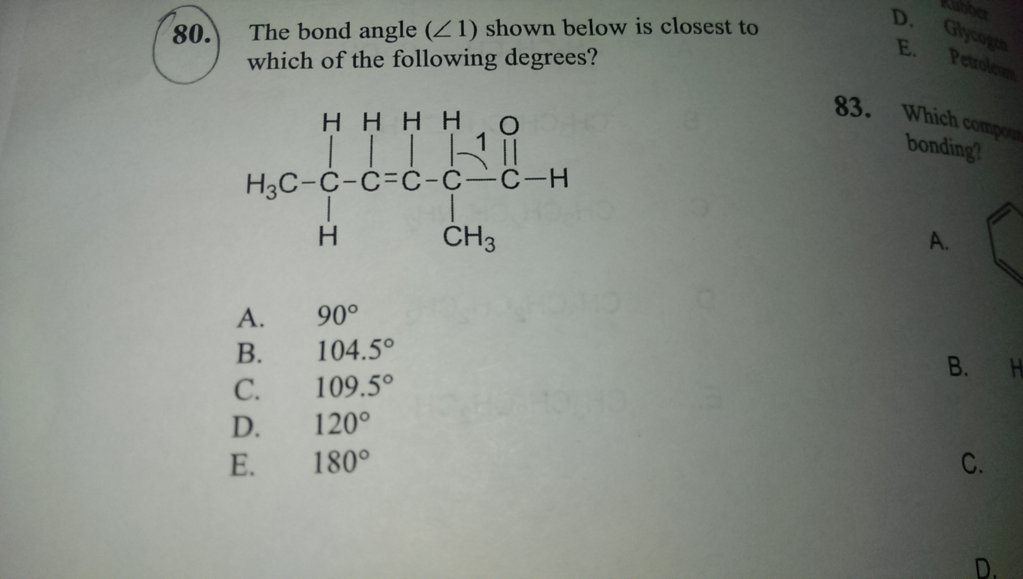
I thought that the carbon was sp3 hybridized, as it is attached to 3 C's and 1 H. Can someone please help me figure this out? I answered that the bond angle would be 109.5 for sp3, but the answer is 120. Why is this sp2? Thank you!