How are you able to distinguish between the two when answering a question?
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
What's the difference between thermodynamically favored vs. Kinetically favored?
- Thread starter Mr. otcoD
- Start date
- Joined
- Feb 16, 2016
- Messages
- 594
- Reaction score
- 96
Do you have a specific question that pertains to your problem?
It depends on the experimental conditions. Usually, if you have a kinetic product, you can convert it into the thermodynamic product if you raise the temperature of the reaction. Conversely, if you raise the temp and the product remains the same, then it is likely that you already have the thermodynamic product.
If given a reaction coordinate, you should easily be able to identify these two.
If given a reaction coordinate, you should easily be able to identify these two.
I took NS8 today and this was what I came across. I couldn't decide between thermo favorably vs. kinetically favorably.
I just chose kinetically because it gave me a graph regarding a reaction (transitions states and everything. How the line was literally below the product line (indicating that its SO easy to reach that complex)
Is the answer literally just because its exothermic?
I just chose kinetically because it gave me a graph regarding a reaction (transitions states and everything. How the line was literally below the product line (indicating that its SO easy to reach that complex)
Is the answer literally just because its exothermic?
Attachments
- Joined
- Feb 16, 2016
- Messages
- 594
- Reaction score
- 96
Like aldol said, kinetic is favored with low temp and thermo is with hi temp. Seems that the one at a lower energy is the thermodynamic one because it's more stable.
I'm assuming the y axis is free energy and not enthalpy, so I don't think it would necessarily mean it's exothermic.
@aldol16 am I right?
I'm assuming the y axis is free energy and not enthalpy, so I don't think it would necessarily mean it's exothermic.
@aldol16 am I right?
- Joined
- Mar 23, 2014
- Messages
- 2,127
- Reaction score
- 2,276
Alright, so this question does not have to do with kinetic vs. thermodynamic product. Kinetic and thermodynamic products are only applicable when you have a partitioning between two possible products and one product faces a lower activation energy to get to but is higher in energy than the other product.
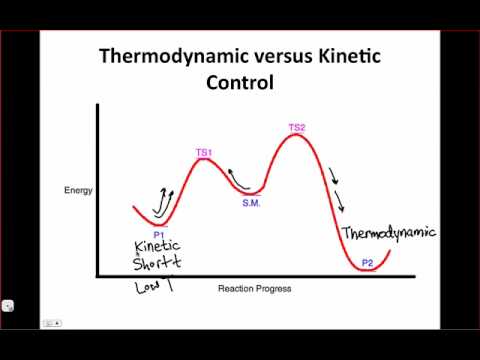
So in this picture, start off in the middle. To go left, it's easier and requires less energy. To go right requires more activation energy. However, the product on the right is more stable. So if you wanted the kinetic product, you'd do this reaction at low temperatures so that the system has enough energy to overcome the activation barrier on the left but not that on the right. This is why, if you want a kinetic enolate, you do it at -78 or something. If you want the thermodynamic product, you just heat it all up and the whole system will shift towards the thermodynamic product.
Okay, so for this question, there is no kinetic product. There is just one reaction with one product, with a lot of intermediates in between. All the BHT is doing here is lowering the energy of one of the intermediates such that the next activation energy (to be traversed en route to the product from that intermediate) is so high that the intermediate is stuck in a potential well and cannot escape. Thus, the peroxide product is never reached.

So in this picture, start off in the middle. To go left, it's easier and requires less energy. To go right requires more activation energy. However, the product on the right is more stable. So if you wanted the kinetic product, you'd do this reaction at low temperatures so that the system has enough energy to overcome the activation barrier on the left but not that on the right. This is why, if you want a kinetic enolate, you do it at -78 or something. If you want the thermodynamic product, you just heat it all up and the whole system will shift towards the thermodynamic product.
Okay, so for this question, there is no kinetic product. There is just one reaction with one product, with a lot of intermediates in between. All the BHT is doing here is lowering the energy of one of the intermediates such that the next activation energy (to be traversed en route to the product from that intermediate) is so high that the intermediate is stuck in a potential well and cannot escape. Thus, the peroxide product is never reached.
- Joined
- Nov 15, 2014
- Messages
- 18
- Reaction score
- 1
So just to clarify, is the question basically just asking whether the intermediate is more kinetically favorable versus thermodynamically favorable THAN the product (peroxide) itself? And if this is the case, the intermediate is more thermodynamically favorable than the product because it sits at a lower energy state?
So just to clarify, is the question basically just asking whether the intermediate is more kinetically favorable versus thermodynamically favorable THAN the product (peroxide) itself? And if this is the case, the intermediate is more thermodynamically favorable than the product because it sits at a lower energy state?
The question is only talking about thermodynamics and not about kinetics. So it's asking whether the intermediate is more stable than the product itself.
Similar threads
- Replies
- 2
- Views
- 627
D
- Replies
- 1
- Views
- 3K
- Replies
- 2
- Views
- 505
- Replies
- 1
- Views
- 7K
- Question
- Replies
- 1
- Views
- 764

