- Joined
- Jan 5, 2011
- Messages
- 173
- Reaction score
- 1
does benzene produce a higher or lower heat of hydrogenation than expected? i said benzene gives a lower than expected heat of hydrogenation because it is so stable but they say the opposite. (that it gives a higher than expected)
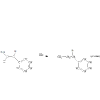
and also, would The reaction of 1-phenyl-1-propene with HBr give
A) 2-bromo-1-phenylpropane
B) 1-bromo-1-phenylpropane
i say 1 bromo but they say it's A.
and also, would The reaction of 1-phenyl-1-propene with HBr give
A) 2-bromo-1-phenylpropane
B) 1-bromo-1-phenylpropane
i say 1 bromo but they say it's A.