- Joined
- Dec 6, 2014
- Messages
- 45
- Reaction score
- 110
Hi everyone, I was wondering if someone could explain which proton is more acidic for this problem:
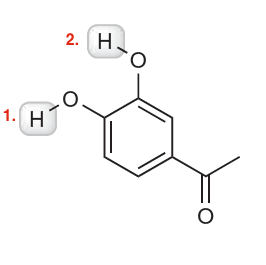
Also, for the next problem, is this an exception to the CARDIO rule? Would proton #2 be more acidic due to the sp character even though proton #1 has resonance structures?
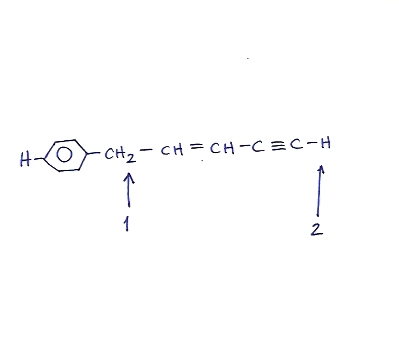
Also, for the next problem, is this an exception to the CARDIO rule? Would proton #2 be more acidic due to the sp character even though proton #1 has resonance structures?
