- Joined
- Feb 3, 2010
- Messages
- 459
- Reaction score
- 2
Why does acidity increase as you go down a group?
If a compound can donate protons or accept electrons (and lower pH) it's considered acidic correct? Doesn't electron affinity become more positive as you go down a group? And isn't electron affinity a measure of the energy released/required to add electrons?
Also.. I wanted to clarify the trends I have been memorizing:
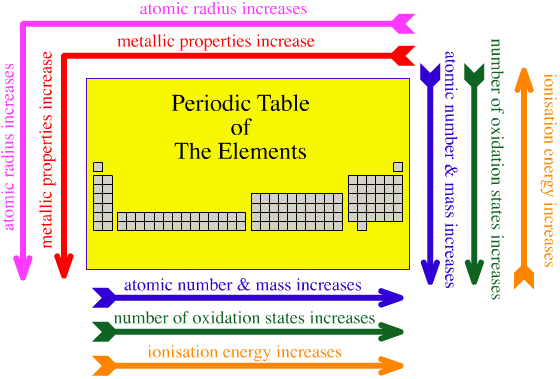
Atomic Radius: Increases RIGHT TO LEFT a period and DOWN a group.
Electronegativity: Increases LEFT TO RIGHT a period and UP a group.
Electron Affinity: Becomes more negative LEFT TO RIGHT a period and UP a group.
Ionization Energy: Increases LEFT TO RIGHT a period and UP a group (with 2nd I.E > 1st I.E)
Acidity: Increases LEFT TO RIGHT a period and DOWN a group?
If a compound can donate protons or accept electrons (and lower pH) it's considered acidic correct? Doesn't electron affinity become more positive as you go down a group? And isn't electron affinity a measure of the energy released/required to add electrons?
Also.. I wanted to clarify the trends I have been memorizing:
Atomic Radius: Increases RIGHT TO LEFT a period and DOWN a group.
Electronegativity: Increases LEFT TO RIGHT a period and UP a group.
Electron Affinity: Becomes more negative LEFT TO RIGHT a period and UP a group.
Ionization Energy: Increases LEFT TO RIGHT a period and UP a group (with 2nd I.E > 1st I.E)
Acidity: Increases LEFT TO RIGHT a period and DOWN a group?