- Joined
- Apr 7, 2011
- Messages
- 5,313
- Reaction score
- 1,085
http://www.nejm.org/doi/full/10.1056/NEJMp1511754
At times, the Food and Drug Administration (FDA) must grapple with safety concerns related to off-label uses of FDA-approved medications. Over the past several years, we have sought to understand the risk of serious neurologic events that occur after the epidural injection of glucocorticoids (corticosteroids) — a procedure that is commonly performed in the United States in an effort to manage radicular neck and back pain. The FDA has not approved any injectable glucocorticoid product for epidural administration, so any such use is considered off-label — part of the practice of medicine and not regulated by the FDA.
In 2009, the FDA began evaluating serious neurologic events associated with epidural glucocorticoid injections. Between 1997 and 2014, a total of 90 serious and sometimes fatal neurologic events were reported to the FDA Adverse Event Reporting System (FAERS), including cases of paraplegia, quadriplegia, spinal cord infarction, and stroke. (Compounded glucocorticoids used in epidural injections have been associated with fungal meningitis, but cases involving contaminated products were not included in the case series under consideration.) Potential causes of these adverse events included technique-related problems such as intrathecal injection, epidural hematoma, direct spinal cord injury, and embolic infarction after inadvertent intraarterial injection.
Procedure- and patient-related factors may contribute to the risk of serious neurologic events. These factors include the level of spinal injection, the method of approaching the epidural space (e.g., interlaminar or transforaminal), and the degree of sedation of the patient. For example, the vascular anatomy of the cervical region may increase the risk of inadvertent intraarterial injection with the transforaminal approach. Although radiographic guidance may help minimize risk, there are reports in FAERS of serious neurologic events even with the use of fluoroscopy. Also, patients who are more heavily sedated during the procedure may not be able to provide feedback about symptoms caused by inadvertent contact with neural structures.
A central question is the role of the glucocorticoids themselves in these adverse events. There is concern that in glucocorticoids formulated as suspensions rather than solutions, particulate matter may pose an increased risk of embolism after inadvertent intravascular injection. All catastrophic events (those resulting in permanent disability or death) reported to FAERS were associated with injection of a suspension, whereas only a few cases involving temporary symptoms were reported with glucocorticoid solutions.
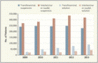
To evaluate the relative use in the United States of these different glucocorticoid formulations in epidural injections, the FDA analyzed health care claims data from IMS Health (projected to the U.S. commercially insured population) and Medicare. Among beneficiaries of Medicare Parts A and B who were 65 years of age or older in 2013, more than 1.3 million epidural glucocorticoid injections were performed in approximately 426,000 patients. IMS Health data indicate that an estimated 604,000 commercially insured U.S. patients less than 65 years of age received an epidural glucocorticoid injection in 2013 Estimated Numbers of Patients under 65 Years of Age in the Commercially Insured U.S. Population Who Received an Epidural Glucocorticoid Injection (EGI), According to Method of Administration and Type of Formulation, 2009–2013.). Although we do not have access to information about the extent of utilization of compounded products used for such injections because they are not regulated by the FDA, Medicare and IMS Health data show that suspension formulations accounted for more than 80% of utilization of commercially available products. Because the use of solutions is so limited, it's difficult to assess the relative safety of solutions as compared with suspensions on the basis of the existing data. The increasing use of solutions for epidural injections by the transforaminal approach between 2009 and 2013, from 5% to 15% of patients younger than 65, may reflect increased concern regarding the safety of suspensions administered by the transforaminal route (see graph). A similar but smaller trend was observed in the Medicare population (an increase from 4% to 9%).
Although inadvertent intraarterial injection is one mechanism for serious neurologic events, there are other potential causes. A study conducted as part of the American Society of Anesthesiologists' Closed Claims Project showed that in cases of cervical procedures for chronic pain that led to malpractice claims, direct needle trauma to a nerve or the spinal cord was the most common procedure-related event.1 Although many observers believe that the risk of injury occurs primarily with transforaminal cervical injections, the authors of this analysis found that of the cervical epidural procedures that were associated with spinal cord injury, two thirds were performed with the interlaminar approach and one third with the transforaminal approach.
Because of the technical nature of epidural glucocorticoid injection, in 2011 the FDA's Safe Use Initiative facilitated the organization of an external expert working group to engage the community that performs these procedures in developing recommendations for minimizing the risk of serious neurologic events. That group recently published its clinical considerations for health care providers.2 Although the FDA facilitated meetings, the recommendations come from the working group, not the agency.
In 2014, the FDA issued a requirement that all injectable glucocorticoid product labels carry a warning stating that “serious neurologic events, some resulting in death, have been reported with epidural injection of corticosteroids” and that the “safety and effectiveness of epidural administration of corticosteroids have not been established and corticosteroids are not approved for this use.”3 The agency determined that the class warning was warranted on the basis of its analysis of FAERS cases and reports in the medical literature of serious neurologic events. The warning did not distinguish any difference in the risk associated with the various injection approaches (interlaminar, transforaminal, and caudal), locations of spinal injection (cervical, thoracic, lumbar, and sacral), or glucocorticoid formulations (solutions and suspensions), because the data suggested that each approach, location, and formulation was associated with some risk of neurologic injury.
We believe that it is important to warn patients and practitioners about the risk of these serious, albeit rare, adverse events and to remind providers that epidural injection is an off-label use of glucocorticoids.
The FDA held an advisory committee meeting in November 2014 to obtain external expert input on this matter and to discuss whether further regulatory actions were necessary. One key question for the committee was whether a contraindication was warranted to restrict the injection of glucocorticoids into the epidural space. Before and during the advisory committee meeting, the FDA received feedback regarding the scope of the class warning. There was a wide range of opinions, from support for stronger labeling, including such elements as a contraindication, to arguments that the warning was too broad and should focus on particular approaches, spinal regions, formulations, or some combination thereof. Many advisory committee members expressed concern about the risk of cervical transforaminal injection of suspension glucocorticoid formulas and recommended that the FDA contraindicate suspension products for this use. Some also thought that the FDA should modify its statement to say that safety and effectiveness of the injections have not been established “by the FDA.”
After carefully considering the feedback provided at the advisory committee meeting, the FDA has decided not to modify the warning about serious neurologic events. Without question, serious (sometimes fatal) neurologic events occur with epidural glucocorticoid injection. Given the large number of these procedures performed, these events appear to be rare; however, a population-based study would be needed to establish a valid estimate of their frequency. We find that available data do not currently support either a contraindication or a warning focused only on cervical transforaminal injection of suspension glucocorticoids. Although many experts believe the risk is greatest with suspensions, the available data do not support comparative safety labeling implying that solutions are safer. Such labeling could encourage practitioners to use solutions, even though their relative safety and effectiveness remain an open question. Regarding effectiveness, some published studies support the benefit of epidural glucocorticoid injection,4 but others call that benefit into question.5 Patient selection may be the key to optimizing the efficacy of epidural glucocorticoid injection, and we encourage the medical community to work to identify the types of patients who might benefit most.
At times, the Food and Drug Administration (FDA) must grapple with safety concerns related to off-label uses of FDA-approved medications. Over the past several years, we have sought to understand the risk of serious neurologic events that occur after the epidural injection of glucocorticoids (corticosteroids) — a procedure that is commonly performed in the United States in an effort to manage radicular neck and back pain. The FDA has not approved any injectable glucocorticoid product for epidural administration, so any such use is considered off-label — part of the practice of medicine and not regulated by the FDA.
In 2009, the FDA began evaluating serious neurologic events associated with epidural glucocorticoid injections. Between 1997 and 2014, a total of 90 serious and sometimes fatal neurologic events were reported to the FDA Adverse Event Reporting System (FAERS), including cases of paraplegia, quadriplegia, spinal cord infarction, and stroke. (Compounded glucocorticoids used in epidural injections have been associated with fungal meningitis, but cases involving contaminated products were not included in the case series under consideration.) Potential causes of these adverse events included technique-related problems such as intrathecal injection, epidural hematoma, direct spinal cord injury, and embolic infarction after inadvertent intraarterial injection.
Procedure- and patient-related factors may contribute to the risk of serious neurologic events. These factors include the level of spinal injection, the method of approaching the epidural space (e.g., interlaminar or transforaminal), and the degree of sedation of the patient. For example, the vascular anatomy of the cervical region may increase the risk of inadvertent intraarterial injection with the transforaminal approach. Although radiographic guidance may help minimize risk, there are reports in FAERS of serious neurologic events even with the use of fluoroscopy. Also, patients who are more heavily sedated during the procedure may not be able to provide feedback about symptoms caused by inadvertent contact with neural structures.
A central question is the role of the glucocorticoids themselves in these adverse events. There is concern that in glucocorticoids formulated as suspensions rather than solutions, particulate matter may pose an increased risk of embolism after inadvertent intravascular injection. All catastrophic events (those resulting in permanent disability or death) reported to FAERS were associated with injection of a suspension, whereas only a few cases involving temporary symptoms were reported with glucocorticoid solutions.
To evaluate the relative use in the United States of these different glucocorticoid formulations in epidural injections, the FDA analyzed health care claims data from IMS Health (projected to the U.S. commercially insured population) and Medicare. Among beneficiaries of Medicare Parts A and B who were 65 years of age or older in 2013, more than 1.3 million epidural glucocorticoid injections were performed in approximately 426,000 patients. IMS Health data indicate that an estimated 604,000 commercially insured U.S. patients less than 65 years of age received an epidural glucocorticoid injection in 2013 Estimated Numbers of Patients under 65 Years of Age in the Commercially Insured U.S. Population Who Received an Epidural Glucocorticoid Injection (EGI), According to Method of Administration and Type of Formulation, 2009–2013.). Although we do not have access to information about the extent of utilization of compounded products used for such injections because they are not regulated by the FDA, Medicare and IMS Health data show that suspension formulations accounted for more than 80% of utilization of commercially available products. Because the use of solutions is so limited, it's difficult to assess the relative safety of solutions as compared with suspensions on the basis of the existing data. The increasing use of solutions for epidural injections by the transforaminal approach between 2009 and 2013, from 5% to 15% of patients younger than 65, may reflect increased concern regarding the safety of suspensions administered by the transforaminal route (see graph). A similar but smaller trend was observed in the Medicare population (an increase from 4% to 9%).
Although inadvertent intraarterial injection is one mechanism for serious neurologic events, there are other potential causes. A study conducted as part of the American Society of Anesthesiologists' Closed Claims Project showed that in cases of cervical procedures for chronic pain that led to malpractice claims, direct needle trauma to a nerve or the spinal cord was the most common procedure-related event.1 Although many observers believe that the risk of injury occurs primarily with transforaminal cervical injections, the authors of this analysis found that of the cervical epidural procedures that were associated with spinal cord injury, two thirds were performed with the interlaminar approach and one third with the transforaminal approach.
Because of the technical nature of epidural glucocorticoid injection, in 2011 the FDA's Safe Use Initiative facilitated the organization of an external expert working group to engage the community that performs these procedures in developing recommendations for minimizing the risk of serious neurologic events. That group recently published its clinical considerations for health care providers.2 Although the FDA facilitated meetings, the recommendations come from the working group, not the agency.
In 2014, the FDA issued a requirement that all injectable glucocorticoid product labels carry a warning stating that “serious neurologic events, some resulting in death, have been reported with epidural injection of corticosteroids” and that the “safety and effectiveness of epidural administration of corticosteroids have not been established and corticosteroids are not approved for this use.”3 The agency determined that the class warning was warranted on the basis of its analysis of FAERS cases and reports in the medical literature of serious neurologic events. The warning did not distinguish any difference in the risk associated with the various injection approaches (interlaminar, transforaminal, and caudal), locations of spinal injection (cervical, thoracic, lumbar, and sacral), or glucocorticoid formulations (solutions and suspensions), because the data suggested that each approach, location, and formulation was associated with some risk of neurologic injury.
We believe that it is important to warn patients and practitioners about the risk of these serious, albeit rare, adverse events and to remind providers that epidural injection is an off-label use of glucocorticoids.
The FDA held an advisory committee meeting in November 2014 to obtain external expert input on this matter and to discuss whether further regulatory actions were necessary. One key question for the committee was whether a contraindication was warranted to restrict the injection of glucocorticoids into the epidural space. Before and during the advisory committee meeting, the FDA received feedback regarding the scope of the class warning. There was a wide range of opinions, from support for stronger labeling, including such elements as a contraindication, to arguments that the warning was too broad and should focus on particular approaches, spinal regions, formulations, or some combination thereof. Many advisory committee members expressed concern about the risk of cervical transforaminal injection of suspension glucocorticoid formulas and recommended that the FDA contraindicate suspension products for this use. Some also thought that the FDA should modify its statement to say that safety and effectiveness of the injections have not been established “by the FDA.”
After carefully considering the feedback provided at the advisory committee meeting, the FDA has decided not to modify the warning about serious neurologic events. Without question, serious (sometimes fatal) neurologic events occur with epidural glucocorticoid injection. Given the large number of these procedures performed, these events appear to be rare; however, a population-based study would be needed to establish a valid estimate of their frequency. We find that available data do not currently support either a contraindication or a warning focused only on cervical transforaminal injection of suspension glucocorticoids. Although many experts believe the risk is greatest with suspensions, the available data do not support comparative safety labeling implying that solutions are safer. Such labeling could encourage practitioners to use solutions, even though their relative safety and effectiveness remain an open question. Regarding effectiveness, some published studies support the benefit of epidural glucocorticoid injection,4 but others call that benefit into question.5 Patient selection may be the key to optimizing the efficacy of epidural glucocorticoid injection, and we encourage the medical community to work to identify the types of patients who might benefit most.