- Joined
- Aug 15, 2014
- Messages
- 34
- Reaction score
- 3
What is the molar solubility of BaF2 (Ksp = 1.8×10-7) in 0.10 M sodium fluoride?
Solution by bootcamp:
BaF2(s)
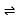 Ba2+(aq) + 2F-(aq)
Ba2+(aq) + 2F-(aq)
Ksp = [Ba2+][F-]2
We can solve this via an ICE table, which is the longer way, or we can skip to the end and just use the Ksp equation above to solve the question. Speed is important on the DAT, so we’re going to solve this the fast way.
Let x = molar solubility of BaF2
We have 1 ion of Ba2+ and 2 ions of F-. We’re going to assume that the molar solubility of BaF2 is very small (common on the DAT since we don’t have a calculator) and that it probably won’t impact the concentration of fluoride that much. Therefore:
Ksp = [Ba2+][F-]2
Ksp = 1.8×10-7 = (x)(0.10)2
Shouldn't the [F] solubility be 2x^2??
Solution by bootcamp:
BaF2(s)
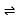
Ksp = [Ba2+][F-]2
We can solve this via an ICE table, which is the longer way, or we can skip to the end and just use the Ksp equation above to solve the question. Speed is important on the DAT, so we’re going to solve this the fast way.
Let x = molar solubility of BaF2
We have 1 ion of Ba2+ and 2 ions of F-. We’re going to assume that the molar solubility of BaF2 is very small (common on the DAT since we don’t have a calculator) and that it probably won’t impact the concentration of fluoride that much. Therefore:
Ksp = [Ba2+][F-]2
Ksp = 1.8×10-7 = (x)(0.10)2
Shouldn't the [F] solubility be 2x^2??
