avalonisland888
Full Member
- Joined
- May 22, 2020
- Messages
- 60
- Reaction score
- 7
- Points
- 0
- Pre-Medical
Advertisement - Members don't see this ad
1) I'm confused about how we got to the ratio Kd = forward/reverse. This is what went through my head when trying to reason out the question.
If the reaction is A --> B ( forward is a dissociation rxn)
The rate constant for the forward is k[A] whereas the rate constant for the reverse is kB
The equilibrium constant is products/reactants = concentration of B/concentration of A
And now I'm stuck because I don't know how equilibrium constant became kA/kB
2) Also, if the question was asking for the association constant Ka, we would just take the inverse of the dissociation constant Kd right? Please help!!
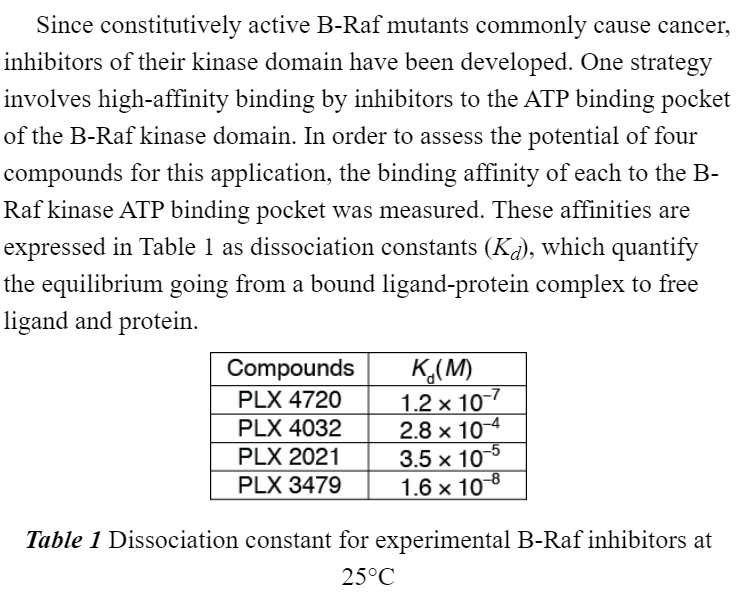
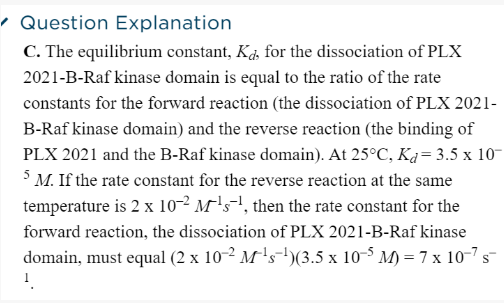
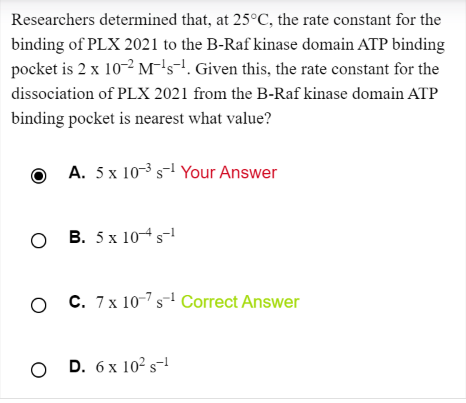
If the reaction is A --> B ( forward is a dissociation rxn)
The rate constant for the forward is k[A] whereas the rate constant for the reverse is kB
The equilibrium constant is products/reactants = concentration of B/concentration of A
And now I'm stuck because I don't know how equilibrium constant became kA/kB
2) Also, if the question was asking for the association constant Ka, we would just take the inverse of the dissociation constant Kd right? Please help!!

