You might not be understanding the difference between pKa and pH.
You can't ask at a pKa of 6, would it be protonated/deprotonated...
pKa is the measurement of how strong the acid is, pH is the measurement of how acidic or basic a sovlent is.
pKa is like what you were born with, and pH is like environmental factors.
The questions is that the "environment" is pH=7, would His be protonated (HA) or deprotonated (A-)
Just like everyone has been replying. Given that His pKa=6 @ pH=7; When pH>pKa (7>6)=we know that its in the deprotonated form=A- (no H+ attached)
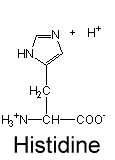
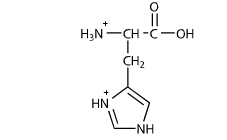
this is the deprotonated form pKa(6)<pH=neutral; we can assume that this probably exists in a solution pH=7+
this is the protonated form=exists only when pKa(6)>pH=+1 charge; we can assume that this probably exists in a solution pH=0-6
Another way to remember pH><pKa is to connect the dots of pH vs. [H+]
when pH is low=[H+] is high meaning a lot of H+ ions are floating around.
So when pH<pKa (pH is low), we can assume that H+ ions are high concentration wanting to protonate something. so that molecule must be protonated.
therefore, when pH<pKa, [H+] High, molecule in protonated state.
Hope this helps. You can look up all the amino acid chart pKa's and practice at different pHs drawing whether the structure will be deprotonated or protonated...