- Joined
- Feb 13, 2014
- Messages
- 15
- Reaction score
- 0
- Points
- 4,571
- DO/PhD Student
When we look at conjugated systems, systems with delocalized electrons, we have to have two different P orbitals connecting across a sigma bond. This is easy enough to see in a conjugated diene.

But we also get a conjugated system when we have an allylic 1) Radical 2) Anion 3) Cation 4) Lone Pair
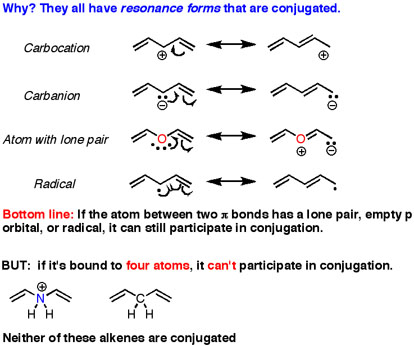
Here's where I'm confused
In the case of the lone pair/anion, aren't these lone pair of electrons in an SP3, NOT a P orbital?
How can they contribute to a conjugated system if they themselves aren't in P orbitals?

But we also get a conjugated system when we have an allylic 1) Radical 2) Anion 3) Cation 4) Lone Pair

Here's where I'm confused
In the case of the lone pair/anion, aren't these lone pair of electrons in an SP3, NOT a P orbital?
How can they contribute to a conjugated system if they themselves aren't in P orbitals?
