- Joined
- Feb 10, 2011
- Messages
- 895
- Reaction score
- 126
- Points
- 4,746
I saw that alpha is if the C1 - OH group is down (ie. trans to the CH2OH subsituent at C5 which always points up ) or beta if C1-OH is up (cis to the CH2OH substiuent on C5 carbon which always point up).
The TBR says this too but didn't find the explainations too through.
Wikipedia says the following:
α-D-Glucopyranose has opposite stereochemistry at both C-1 and C-5 (respectively S and R), whereas in β-D-glucopyranose they are the same (both R). <---COOL , make sense as said above.
In α-L-arabinopyranose, the absolute configurations at C-1 and C-4 are respectively R and S, so they are also different, but opposite to those in α-D-glucopyranose. Both sugars are α, even though the position of the anomeric oxygen is different.<---WOah, what happened here? Is it b/c we dont see the position (equi or axial up or down) of the subsituents on C5?
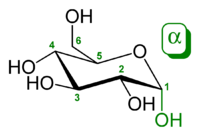

α-D-glucopyranoseβ-D-glucopyranose
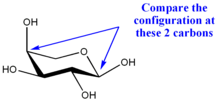
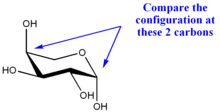
α-L-arabinopyranoseβ-L-arabinopyranose
The TBR says this too but didn't find the explainations too through.
Wikipedia says the following:
α-D-Glucopyranose has opposite stereochemistry at both C-1 and C-5 (respectively S and R), whereas in β-D-glucopyranose they are the same (both R). <---COOL , make sense as said above.
In α-L-arabinopyranose, the absolute configurations at C-1 and C-4 are respectively R and S, so they are also different, but opposite to those in α-D-glucopyranose. Both sugars are α, even though the position of the anomeric oxygen is different.<---WOah, what happened here? Is it b/c we dont see the position (equi or axial up or down) of the subsituents on C5?


α-D-glucopyranoseβ-D-glucopyranose


α-L-arabinopyranoseβ-L-arabinopyranose
