- Joined
- Mar 9, 2017
- Messages
- 233
- Reaction score
- 9
In typical glycoside-link hydrolysis, is there a rule to whether the OH from water goes to the sugar on the left of the disaccharide or to the right side monosaccharide? When water hydrolyzes the glycoside link, one of the sugars in the link will receive an OH, and the other will receive the H, while the opposite occurs when the link is formed via condensation. I posted it again below.
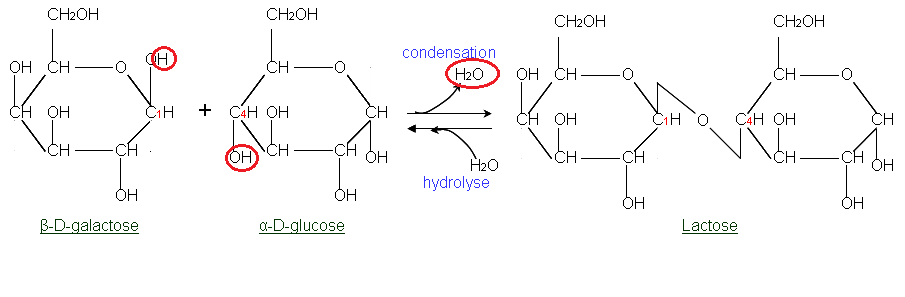
In the example above, will the glucose on the right of the disaccharide above would never receive an OH from water as the bond is lysed, the OH will always go to the galactose?
When two monosaccharides come together, it is due to nucleophilic attack by OH group on an anomeric carbon from 1 sugar on a ring carbon in the other sugar. Typically isn't this attacking sugar placed on the left when drawing the disaccharide, and this is the monosaccharide which would retain its O as the attacking nucleophile? Thus, the sugar that is drawn on the right hand side of the final disaccharide product, lost an OH group. Thus, the sugar on the right would get the OH when hydrolyzing the disaccharide (the reverse reaction). Is any of that wrong?
In the example above, will the glucose on the right of the disaccharide above would never receive an OH from water as the bond is lysed, the OH will always go to the galactose?
When two monosaccharides come together, it is due to nucleophilic attack by OH group on an anomeric carbon from 1 sugar on a ring carbon in the other sugar. Typically isn't this attacking sugar placed on the left when drawing the disaccharide, and this is the monosaccharide which would retain its O as the attacking nucleophile? Thus, the sugar that is drawn on the right hand side of the final disaccharide product, lost an OH group. Thus, the sugar on the right would get the OH when hydrolyzing the disaccharide (the reverse reaction). Is any of that wrong?
