- Joined
- Feb 13, 2014
- Messages
- 507
- Reaction score
- 323
- Points
- 5,246
- Pre-Dental
I am extremely confused and hoping someone might be able to help me figure this one out. Considering the two reactions below:
In the first reaction, the hot KMnO4 only oxidizes the methyl group. Why doesn't it cleave the double bonds in the ring? It cleaves the ring's double bond in the second reaction, so why not in this first?
REACTION 1:
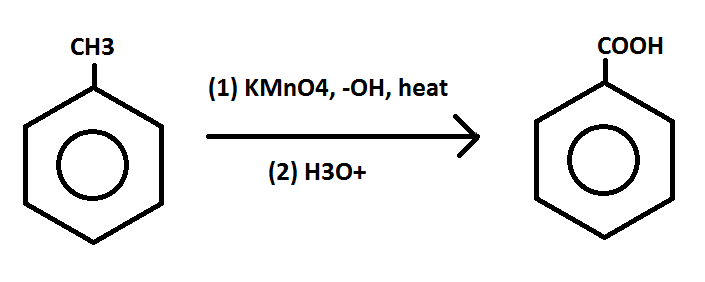
REACTION 2:
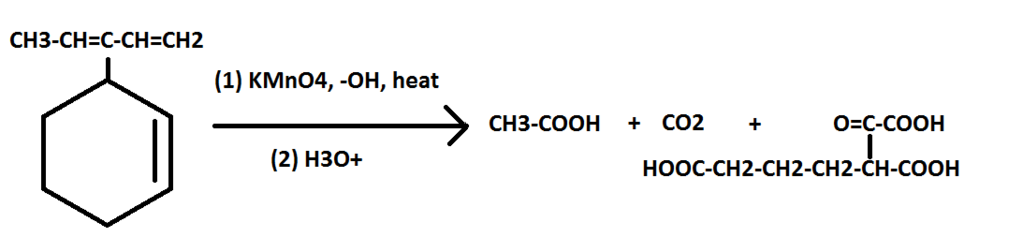
I have a feeling I'm missing something very silly, but this has me stumped.
Thank you so much for any responses!!
In the first reaction, the hot KMnO4 only oxidizes the methyl group. Why doesn't it cleave the double bonds in the ring? It cleaves the ring's double bond in the second reaction, so why not in this first?
REACTION 1:
REACTION 2:
I have a feeling I'm missing something very silly, but this has me stumped.
Thank you so much for any responses!!
