- Joined
- Nov 8, 2013
- Messages
- 125
- Reaction score
- 23
- Points
- 4,671
- Pre-Dental
I never seem to get the best resonance structure right! help??? any suggestions, tips?

Specifically, what's the problem? Remember, sometimes H's are hidden, and you can't double bond an sp3 Carbon. Does that help?I never seem to get the best resonance structure right! help??? any suggestions, tips?
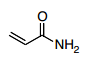
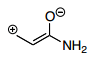
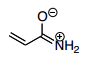
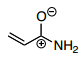
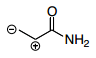
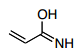
Yikes. E was ruled out immediately due to losing an H. B is the best answer due to conjugation and a non terminal carbocationi would have pulled the trigger on E for its conjugated pi system and never looked back...
good thing i don't need to ever think about organic chemistry ever again
Yikes. E was ruled out immediately due to losing an H. B is the best answer due to conjugation and a non terminal carbocation
I meant that there is no carbocation on a terminal carbon. All the others, except E, which can easily be ruled out due to the Amine group losing a H, have a carbocation. The terminal carbocation would never be a major resonance structure. It's a test taking strategy, that may or may not make sense to anyone else.Which carbocation? We looking at the same B here??
I was thinking more like B because O is more electronegative and therefore can have the neg charge there more than any other, in addition to the octet rule and the pi bonds can move around in diff configurations in that form. N is more likely to to give up an electron so is more likely to be positively charged. C and H has roughly the same so neither one of them wants to be minus or positive charged.thank you everyone 🙂
