- Joined
- Aug 17, 2009
- Messages
- 382
- Reaction score
- 1
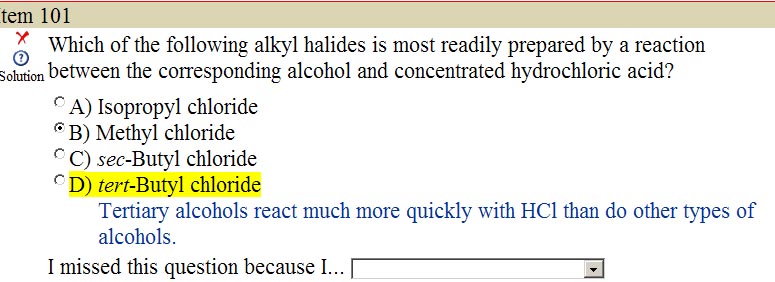
Cl- is a strong nucleophile, so it should proceed via SN2, correct? Their answer presupposes SN1, I'm wondering why??
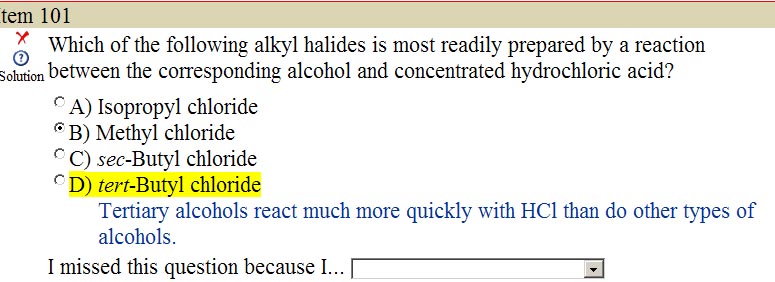
Well
This is a protonated solution which presupposes the SN1
This is a protonated solution which presupposes the SN1

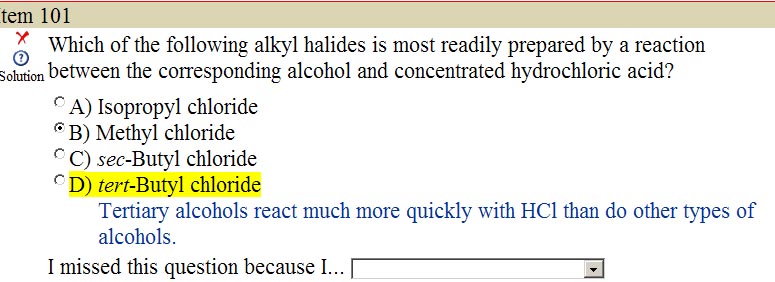
Cl- is a strong nucleophile, so it should proceed via SN2, correct? Their answer presupposes SN1, I'm wondering why??
Yes, thank you. Big learning point for me as well. So I should think "alcohol" = protonated solution = favors SN1= favors most substituted reactant
Thanks.
