There are actually 2 pieces of information that leads to weak than strong.
1. second line has "buffering agent" this automatically rules out B & D. A buffering agent is only a weak acid or weak base.
2. In the second to last line it says the solution is a weakly conducted electricity, meaning it is a weak acid or base, not strong. Strong acids or bases are strong conductors. (review electrolyte/solubility)
3. Since in the passage it says the [H+]=1E-5, then [OH-]=1E-9 because they need to add up to Kw=1E-14
Since [OH-]<[H+], you know this is going to an acidic solution
You are left to choose D.
If you only read the last sentence to infer to D=strong acid, how did you get acid vs. base?
It is also important to understand the difference between dissolve and ionize
A weak acid (ie. vinegar) dissolves in water, but does not completely (100%) ionize.
A strong acid/base does dissolve in water, and does completely ionize.
Pretty much anything will dissolve in water if you give it enough time.
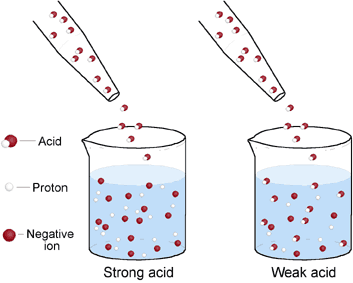
Here is a visual image:
Notice how the weak acid does not completely ionize into H+ and A- like it did in the strong acid.