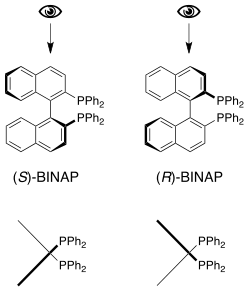
The only thing that might be remotely feasible in terms of the MCAT would be the following question:
(S)-BINAP and (R)-BINAP can best be described as:
a) enantiomers
b) diastereomers,
c) epimers
d) structural isomers
As Lawper said, this would be a hard passage. It would seem that their intent would be to throw you for a loop with the passage and then see if you could recognize the fundamental idea here. With one chiral designation (R or S), they cannot be diastereomers (which require at least two chiral centers) or structure isomers (as seen in the picture, where both structures have identical bonds.) They are non-superimposable mirror images, so they are enantiomers.