D
deleted388502
Hi,
I just want to clear up Question 12 in BR Ochem phase 1 passage 2.
It gives an amide and asks which correctly describes the geometry of the molecule:
A. Nitrogen has trigonal pyramidal geometry, so the two hydrogens are outside of the plane created by the four other atoms.
B. The nitrogen has tetrahedral geometry, so the two hydrogens are outside of the plane created by the other four atoms.
C. The carbon has tetrahedral geometry, so the carbon hydrogen is outside of the plane created by the other five atoms.
D. The six atoms are coplanar
So the answer is D and I would love to hear how someone else approaches this problem. My first thought was that the nitrogen was tetrahedral but I now realize it's between sp2 and sp3, but I don't understand the explanation BR gives. How can the nitrogen be entirely sp2 and trigonal pyramidal?
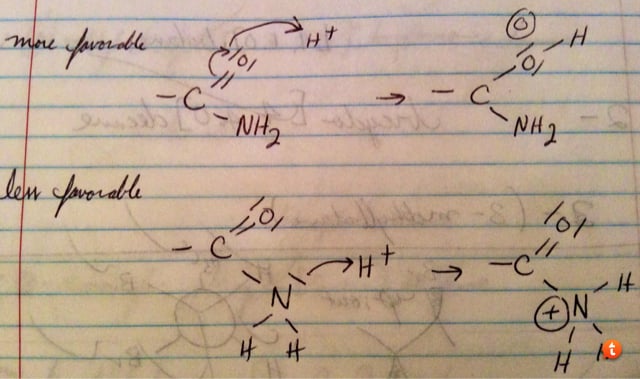
Also, in an amide, the passage has a bunch of questions that talk about how the oxygen is where it's protonated. Why is the oxygen more basic than the nitrogen in an amide? Question 14 says that the most stable hydrogen-bond between amides is from the carbonyl oxygen to the H on the nitrogen - in interpreting this question, why would you choose this answer over the carbonyl oxygen and H on the carbon?
Sorry for all the questions, and I'm greatly appreciative to anyone that can provide insight on this.
Thanks
I just want to clear up Question 12 in BR Ochem phase 1 passage 2.
It gives an amide and asks which correctly describes the geometry of the molecule:
A. Nitrogen has trigonal pyramidal geometry, so the two hydrogens are outside of the plane created by the four other atoms.
B. The nitrogen has tetrahedral geometry, so the two hydrogens are outside of the plane created by the other four atoms.
C. The carbon has tetrahedral geometry, so the carbon hydrogen is outside of the plane created by the other five atoms.
D. The six atoms are coplanar
So the answer is D and I would love to hear how someone else approaches this problem. My first thought was that the nitrogen was tetrahedral but I now realize it's between sp2 and sp3, but I don't understand the explanation BR gives. How can the nitrogen be entirely sp2 and trigonal pyramidal?
Also, in an amide, the passage has a bunch of questions that talk about how the oxygen is where it's protonated. Why is the oxygen more basic than the nitrogen in an amide? Question 14 says that the most stable hydrogen-bond between amides is from the carbonyl oxygen to the H on the nitrogen - in interpreting this question, why would you choose this answer over the carbonyl oxygen and H on the carbon?
Sorry for all the questions, and I'm greatly appreciative to anyone that can provide insight on this.
Thanks
Last edited by a moderator: