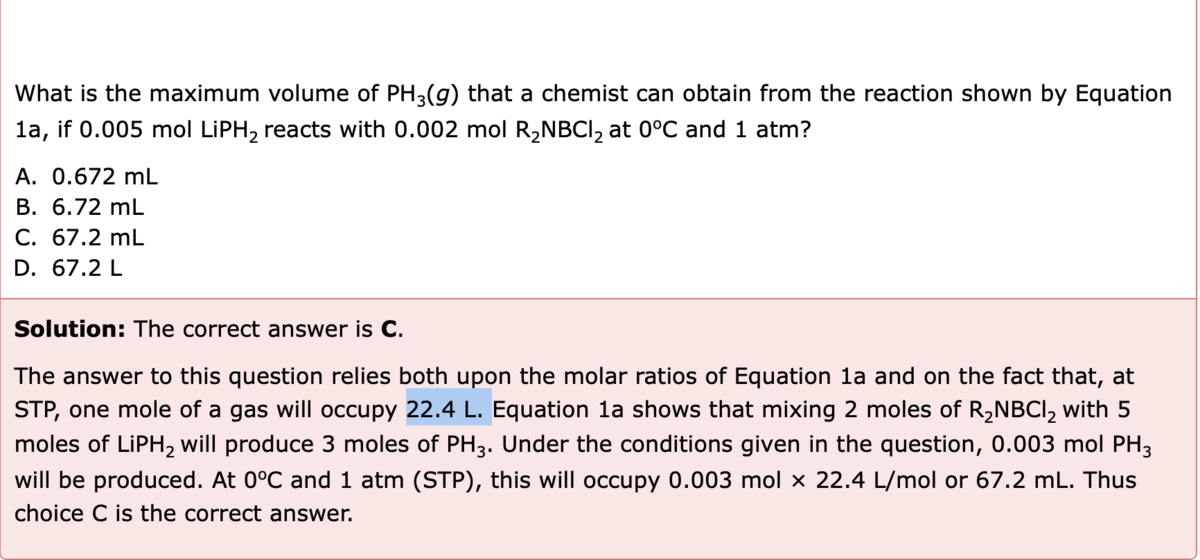
Hi, maybe im overlooking something but can someone explain this to me. Screen shot attached. The way I am looking at it is (.003 mol) x (22.4L/mol), mol cancels out and you get 67.2L or .0672mL. It is saying the answer is 67.2mL. Thank you!
Chem Q-pack question
- Thread starter jmu29
- Start date
