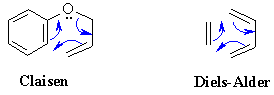
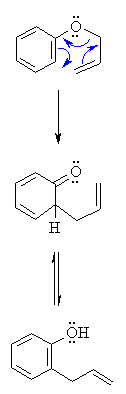
Edit: Aw, fudge. I misread your question as Claisen Condensation. Disregard this. But just really quickly, Diels-Alder is on the outline. Claisen Condensation is very similar to Diels alder. If you understand this, you could probably figure this out as well. This scenario is given a special name because after sigmatropic rearrangement, tautomerization will occur to create a very stable, resonance stablilized structure (a phenol). More than likely it would be explained in a passage:
This is what I wrote previously (thinking you said Claisen Condensation), you can ignore it:
Nope it's not, but if you understand the relative reactivity of carboxylic acid derivatives (where it's electrophilic, stability of LG's) and the fact that carbonyl compounds (Ketones/Aldehydes) can also act as a nucleophile when it's alpha hydrogen is deprotonated, then you should be able to deduce that a nucleophile will attack the electrophile and displace some LG (a common theme in organic). That's all that's really happening in Claisen Condensation. It's given a special name, but all it really is is a reaction between a Ketone enolate and an Ester... viola! It's just a specific way we can form diketones. From there, they can easily ask a whole host of questions. For instance, 1,3-dicarbonyl compounds is on the AAMC outline and they can ask you about keto/enol tautomerization. You should realize in this instance, because of internal hydrogen bonding, the enol form is favored (of course, this is dependent on other factors like the solvent, whether it can engage in resonance, etc), but the generally idea here is applying what you know to unfamiliar situations. If it is presented, don't freak out. Just use what you know and you should be able to reason things out with a clear mind.