- Joined
- Feb 16, 2016
- Messages
- 594
- Reaction score
- 96
A first year graduate student treats the below compound with aq. NaOH and heats the reaction for 1 hour. After cooling down the reaction, what must he do to isolate the desired carboxylic acid product?
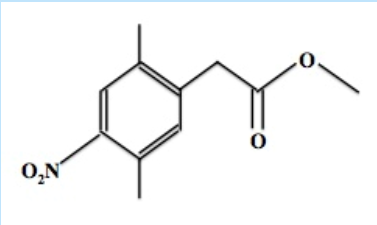
Anybody know how to go about this problem?
Anybody know how to go about this problem?
