Pre-Med Oso
New Member
- Joined
- Feb 22, 2022
- Messages
- 3
- Reaction score
- 0
Hi everyone, I am struggling to understand how the question creators know to use the value 1.5 N H2CO3 in the explanation for this problem. If there is anyone who could explain to me how they got this value it would be much appreciated or if you have a different method of solving these problems please share I would like to know if there is another way to solve the problem.
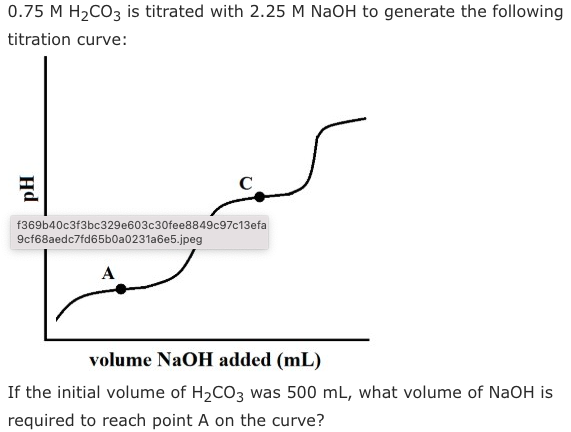
A.83 mL
B.166.5 mL
C.333 mL
D.1000 mL
Answer is A: Point A is the first half-equivalence point, meaning that we need to add exactly half of the volume required to neutralize the first proton, or one-quarter of the amount needed to fully neutralize H2CO3. We can use N1V1 = N2V2: (1.5 N H2CO3)(500 mL) = (2.25 M NaOH)(x mL). Solving for x, we get a volume of 333 mL. However, remember that this equation gives us the volume of base needed to fully neutralize the acid! To answer this question, we need to divide by four, giving us 83.3 mL.
A.83 mL
B.166.5 mL
C.333 mL
D.1000 mL
Answer is A: Point A is the first half-equivalence point, meaning that we need to add exactly half of the volume required to neutralize the first proton, or one-quarter of the amount needed to fully neutralize H2CO3. We can use N1V1 = N2V2: (1.5 N H2CO3)(500 mL) = (2.25 M NaOH)(x mL). Solving for x, we get a volume of 333 mL. However, remember that this equation gives us the volume of base needed to fully neutralize the acid! To answer this question, we need to divide by four, giving us 83.3 mL.

