- Joined
- Feb 4, 2014
- Messages
- 84
- Reaction score
- 2
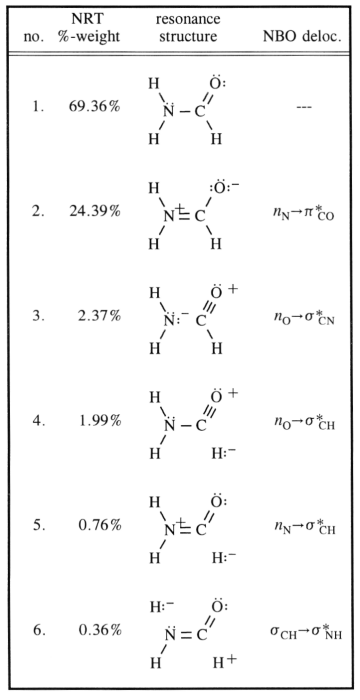
Hello, we know a resonance structure is not valid if say a carbon has 5 bonds. That is more than the octet, and not valid.
But what if a Carbon atom has 3 bonds, no lone pairs, and then a + charge. I saw one example in a textbook where it showed a "valid" resonance structure which had a carbon with 3 bonds and a + charge . But I thought it had to meet the octet, and coming short or having too many bonds was not valid. Why is less than an octet okay, but more than an octet not okay?
Thanks!
But what if a Carbon atom has 3 bonds, no lone pairs, and then a + charge. I saw one example in a textbook where it showed a "valid" resonance structure which had a carbon with 3 bonds and a + charge . But I thought it had to meet the octet, and coming short or having too many bonds was not valid. Why is less than an octet okay, but more than an octet not okay?
Thanks!