I am confused with the derivation of the nernst equation.
My book gives this
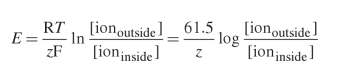
1. If we start with G = Go + RT lnQ and sub in -nFE for G, where is the z (instead of n coming from in the equation?
2. What happens to Eo at the end?
3. I know that Q is Products/Reactants. I am confused with how that translates to ions outside/ions inside.
My book gives this
1. If we start with G = Go + RT lnQ and sub in -nFE for G, where is the z (instead of n coming from in the equation?
2. What happens to Eo at the end?
3. I know that Q is Products/Reactants. I am confused with how that translates to ions outside/ions inside.
