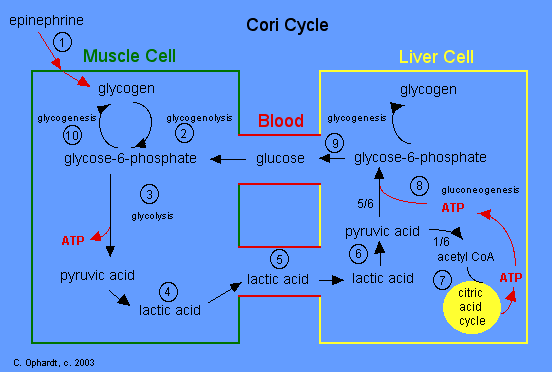
I know that this a controversial topic among biochemists, but how do you guys feel about the concept of lactic acidosis? Looking at any textbook shows that the lactate dehydrogenase reaction actually INCREASES pH by consuming a proton.
This review articles sums things up nicely: http://ajpregu.physiology.org/content/287/3/R502
How many of you still believe that excessive lactate production is a cause of acidosis, rather than an effect? I wanted to get an idea about what the current level of understanding is among anesthesia/ccm people.
This review articles sums things up nicely: http://ajpregu.physiology.org/content/287/3/R502
How many of you still believe that excessive lactate production is a cause of acidosis, rather than an effect? I wanted to get an idea about what the current level of understanding is among anesthesia/ccm people.