- Joined
- Nov 30, 2017
- Messages
- 5
- Reaction score
- 9
There are two reaction mechanism problems that I am having difficulty understanding:
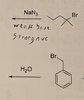
Problem 1: Tertiary halide with azide (N3-) acting as nucleophile/base (solvent is water)
My answer: e1
Key answer: no reaction
My reasoning was that azide would be a strong nucleophile/weak base because it's negatively charged but it is also stabilized by resonance. Since the halide is tertiary we can rule out sn2. We can also rule out E2 because azide is a weak base and Sn1 because azide is a good nucleophile. This leaves us with e1, which I couldn't see any reason to rule out.
Problem 2: Benzylic halide substrate with water acting as the nucleophile/base
My answer: No reaction
Key answer: sn2
My thinking on this one was that it's a primary halide so we can rule out sn1 and e1. We can also rule out elimination because the beta carbon is part of the benzene ring and has no hydrogens to give up. I also ruled out sn2 because water is not a good nucleophile for sn2.
The two problems almost seem to contradict each other. It seems as though problem 2 is saying that the major product will come from an unlikely reaction if all of the other reactions are ruled out. Problem 1 seems to be saying that it won't. Can someone please explain this for me?
Edit: Attached a picture of the two problems
Problem 1: Tertiary halide with azide (N3-) acting as nucleophile/base (solvent is water)
My answer: e1
Key answer: no reaction
My reasoning was that azide would be a strong nucleophile/weak base because it's negatively charged but it is also stabilized by resonance. Since the halide is tertiary we can rule out sn2. We can also rule out E2 because azide is a weak base and Sn1 because azide is a good nucleophile. This leaves us with e1, which I couldn't see any reason to rule out.
Problem 2: Benzylic halide substrate with water acting as the nucleophile/base
My answer: No reaction
Key answer: sn2
My thinking on this one was that it's a primary halide so we can rule out sn1 and e1. We can also rule out elimination because the beta carbon is part of the benzene ring and has no hydrogens to give up. I also ruled out sn2 because water is not a good nucleophile for sn2.
The two problems almost seem to contradict each other. It seems as though problem 2 is saying that the major product will come from an unlikely reaction if all of the other reactions are ruled out. Problem 1 seems to be saying that it won't. Can someone please explain this for me?
Edit: Attached a picture of the two problems