- Joined
- Jun 18, 2013
- Messages
- 362
- Reaction score
- 52
Passage XIII, TBR Ochem section III ...
So the question was
The answer was supposed to be A
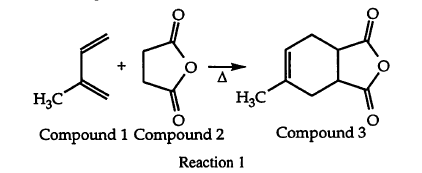
However, doesn't the Diels Alder reaction create different enantiomers preferentially based on endo vs. exo stability? Or does this reaction not display that?
So the question was
The answer was supposed to be A
However, doesn't the Diels Alder reaction create different enantiomers preferentially based on endo vs. exo stability? Or does this reaction not display that?
