Given only a table of time vs. reactant concentration table, how do I figure out the reaction rate, if the reactant concentration difference/time difference is not the same when you use 4 separate pairs of (time , reactant concentration)?
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Reaction rate
- Thread starter m25
- Start date
- Joined
- Feb 27, 2013
- Messages
- 3,469
- Reaction score
- 2,287
@m25 This is a very general question.
Are you taking about using the formula?
ln[conc. at time t] = ln[initial conc.] - k*t
Or if you just mean to use the definition of a rate:
Rate = ΔConcentation/Δt
=(Initial concentration - Final concentration)/Δt
(or just pick any two points and the time between them)
Are you taking about using the formula?
ln[conc. at time t] = ln[initial conc.] - k*t
Or if you just mean to use the definition of a rate:
Rate = ΔConcentation/Δt
=(Initial concentration - Final concentration)/Δt
(or just pick any two points and the time between them)
I think I am talking about the latter(I don't even know the first equation existed), but when I pick between two points I get like 3 and if I choose another two points, I now get 5. So it looks like the time vs concentration graph is not a straight line??@m25 This is a very general question.
Are you taking about using the formula?
ln[conc. at time t] = ln[initial conc.] - k*t
Or if you just mean to use the definition of a rate:
Rate = ΔConcentation/Δt
=(Initial concentration - Final concentration)/Δt
(or just pick any two points and the time between them)
- Joined
- Feb 27, 2013
- Messages
- 3,469
- Reaction score
- 2,287
It is hard to say without seeing the question you are talking about.
But if the rate is dependent on concentration (say first order) then the rate will decrease as the concentration of the reactants decrease.
But if the rate is dependent on concentration (say first order) then the rate will decrease as the concentration of the reactants decrease.
So would theIt is hard to say without seeing the question you are talking about.
But if the rate is dependent on concentration (say first order) then the rate will decrease as the concentration of the reactants decrease.
Rate = ΔConcentation/Δt
only work if the reaction is a first order??
- Joined
- Feb 27, 2013
- Messages
- 3,469
- Reaction score
- 2,287
No.
That is just the definition of rate. It will tell you the overall rate between any two points of data.
The only reactions that have a constant rate are zero order, and then the overall rate can be found using this formula. That is why reactions are described by a rate constant (k) and not an overall rate - for many reactions the rate is not constant.
That is just the definition of rate. It will tell you the overall rate between any two points of data.
The only reactions that have a constant rate are zero order, and then the overall rate can be found using this formula. That is why reactions are described by a rate constant (k) and not an overall rate - for many reactions the rate is not constant.
No.
That is just the definition of rate. It will tell you the overall rate between any two points of data.
The only reactions that have a constant rate are zero order, and then the overall rate can be found using this formula. That is why reactions are described by a rate constant (k) and not an overall rate - for many reactions the rate is not constant.
So say we have a concentration of reactant vs time graph like this:
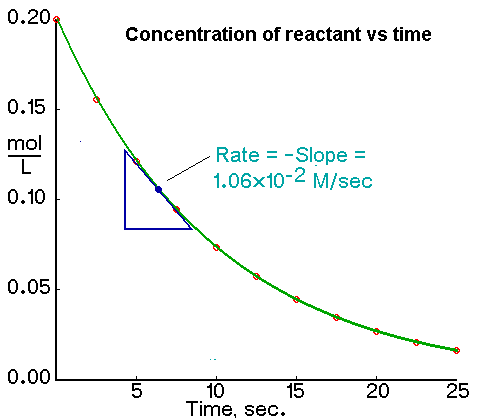
According to the graph, it seems like we can only figure out the INSTANTANEOUS reaction rate as opposed to a general reaction rate for the reaction?
- Joined
- Feb 27, 2013
- Messages
- 3,469
- Reaction score
- 2,287
Right.
Because the rate is changing as the reaction proceeds.
Saying the rate is the negative slope is the same as rate = - ΔConcentation/Δt.
That graph appears to be about first order so rate = k[reactant] and as the [reactant] decreases the rate also decreases.
So if I wanted the rate at t = 3 s I would use a Δt of 2 s and 4s to determine the rate in the middle.
Because the rate is changing as the reaction proceeds.
Saying the rate is the negative slope is the same as rate = - ΔConcentation/Δt.
That graph appears to be about first order so rate = k[reactant] and as the [reactant] decreases the rate also decreases.
So if I wanted the rate at t = 3 s I would use a Δt of 2 s and 4s to determine the rate in the middle.
Oh okay! So if I also wanted to find the constant rate k, then I could first find the reaction rate at t=3 using Δt of 2 s and 4s, and then plug into the equation rxn rate = k[reactant] with [reactant] being the reactant at t=3? And I should get the same constant k along the entire graph, correct?Right.
Because the rate is changing as the reaction proceeds.
Saying the rate is the negative slope is the same as rate = - ΔConcentation/Δt.
That graph appears to be about first order so rate = k[reactant] and as the [reactant] decreases the rate also decreases.
So if I wanted the rate at t = 3 s I would use a Δt of 2 s and 4s to determine the rate in the middle.
But does my approach also work?The best way to find k for a first order reaction is to find the half-life.
Half-life = 0.693/k
so k = 0.693/(half-life)
I am relatively sure that is a formula you should know for the MCAT.
- Joined
- Feb 27, 2013
- Messages
- 3,469
- Reaction score
- 2,287
It may give you an approximation but it is not the easiest or most accurate way. In all likelihood it would be somewhat correct - never tried it.
First order reactions have well established rate constants that be can determined from a simple formula. That would be my approach.
First order reactions have well established rate constants that be can determined from a simple formula. That would be my approach.
So if I don't even know the order of reaction, would my approach still work?It may give you an approximation but it is not the easiest or most accurate way. In all likelihood it would be somewhat correct - never tried it.
First order reactions have well established rate constants that be can determined from a simple formula. That would be my approach.
- Joined
- Feb 27, 2013
- Messages
- 3,469
- Reaction score
- 2,287
If you want the instantaneous rate, then sure. Most of the time you can find the half-life by observation.
Say that in the reaction you posted, the half-life is 6 s (it isn't, I think it is actually about 6.5 but graphs are not perfect). Therefore, k = 0.693/6 s = 0.116 s^-1
Rate when concentration is 0.10 M:
rate = k[conc.] = (.116 s^-1)(0.10 M) = 0.116 M/s
That is pretty close.
Say that in the reaction you posted, the half-life is 6 s (it isn't, I think it is actually about 6.5 but graphs are not perfect). Therefore, k = 0.693/6 s = 0.116 s^-1
Rate when concentration is 0.10 M:
rate = k[conc.] = (.116 s^-1)(0.10 M) = 0.116 M/s
That is pretty close.
Similar threads
- Replies
- 1
- Views
- 858
- Replies
- 2
- Views
- 796
