- Joined
- Dec 29, 2010
- Messages
- 94
- Reaction score
- 0
In going through TBR Physics Chapter 6, i wanted to ensure my understanding is correct, along w/ clarify a few questions
Speed of sound is given by
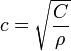
1. v(air) < v(liquid) < v(solid)
This is because the bulk modulus is progressively larger from air to solid, AND the magnitude is far greater than the increase in density. Intuitively, this also makes sense since e.g. in solids the restorative molecular force resisting the compression/rarefaction is larger than in gases.
2. For ideal gases, speed of sound depends only on temp changes
3. Constant temp. Change gas composition
v(lighter gas) > v(heavier gas)
Granted that lighter gas will have lesser density. But, wouldn't it also have lesser restorative force i.e. lesser bulk modulus?
4. Constant gas composition. Change temp
v(higher temp) > v(lesser temp)
High temp --> higher molecular speed --> lesser restorative force? ==> shouldn't speed of sound decrease with increase in temperature?
But, e.g. Q40 asks: If you increase the temp of air by 44%, the speed of sound wave increases by
a. 6.6%
b. 20%
c. 44%
d. 107%
Answer explanation states v proportional to sqrt (temp).
How is this formula arrived at?
Speed of sound is given by
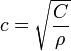
1. v(air) < v(liquid) < v(solid)
This is because the bulk modulus is progressively larger from air to solid, AND the magnitude is far greater than the increase in density. Intuitively, this also makes sense since e.g. in solids the restorative molecular force resisting the compression/rarefaction is larger than in gases.
2. For ideal gases, speed of sound depends only on temp changes
3. Constant temp. Change gas composition
v(lighter gas) > v(heavier gas)
Granted that lighter gas will have lesser density. But, wouldn't it also have lesser restorative force i.e. lesser bulk modulus?
4. Constant gas composition. Change temp
v(higher temp) > v(lesser temp)
High temp --> higher molecular speed --> lesser restorative force? ==> shouldn't speed of sound decrease with increase in temperature?
But, e.g. Q40 asks: If you increase the temp of air by 44%, the speed of sound wave increases by
a. 6.6%
b. 20%
c. 44%
d. 107%
Answer explanation states v proportional to sqrt (temp).
How is this formula arrived at?
Last edited:

