Which of the following lists hydrogen halides in terms of increasing standard heats of formation?
A. HF < HBr < HCl < HI
B. HBr < HF < HCl < HI
C. HF < HI < HCl < HBr
D. HI < HBr < HCl < HF
Answer: A
The general formula for hydrogen halide formation is:
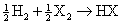 . The reaction that forms the most stable HX will be the most exothermic and have the most negative standard heat of formation. Fluorine is the smallest, most electronegative halogen and forms the only hydrogen halide of the group that is not a strong acid. HF is the most stable hydrogen halide and will have the most negative heat of formation. This eliminates choices B and D. The heat of formation is expected to become increasingly positive as the HX bond length increases (HX bond strength decreases). Indeed, the heat of formation of HI is actually endothermic. This eliminates choice C and makes choice A the best answer.
. The reaction that forms the most stable HX will be the most exothermic and have the most negative standard heat of formation. Fluorine is the smallest, most electronegative halogen and forms the only hydrogen halide of the group that is not a strong acid. HF is the most stable hydrogen halide and will have the most negative heat of formation. This eliminates choices B and D. The heat of formation is expected to become increasingly positive as the HX bond length increases (HX bond strength decreases). Indeed, the heat of formation of HI is actually endothermic. This eliminates choice C and makes choice A the best answer.
Source: TPR GChem Review FSQ Set 9 #5
I understand that answer A is the best option using some POE, but why does the trend show that the standard heat of formation for HBr less than HCl?
If atomic radius increases down the column, then Cl has a shorter bond length than Br? And if electronegativity increases up the column, then Cl is more electronegative than Br? So is there some other factor resulting in HCl having a higher heat of formation than HBr?
Am I making a mistake in comparing atomic radius and bond length?
A. HF < HBr < HCl < HI
B. HBr < HF < HCl < HI
C. HF < HI < HCl < HBr
D. HI < HBr < HCl < HF
Answer: A
The general formula for hydrogen halide formation is:
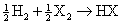
Source: TPR GChem Review FSQ Set 9 #5
I understand that answer A is the best option using some POE, but why does the trend show that the standard heat of formation for HBr less than HCl?
If atomic radius increases down the column, then Cl has a shorter bond length than Br? And if electronegativity increases up the column, then Cl is more electronegative than Br? So is there some other factor resulting in HCl having a higher heat of formation than HBr?
Am I making a mistake in comparing atomic radius and bond length?
