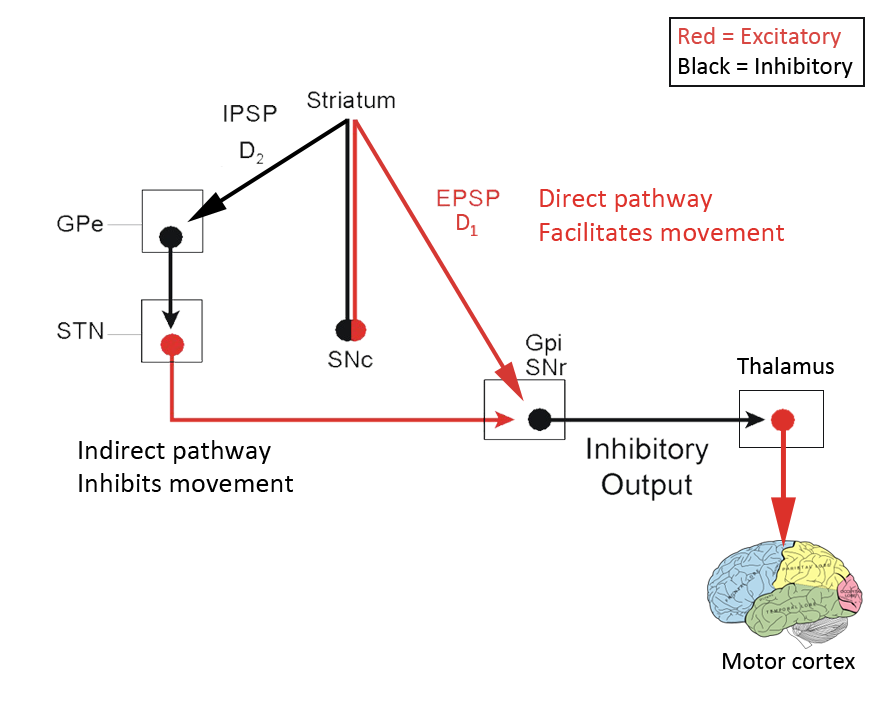
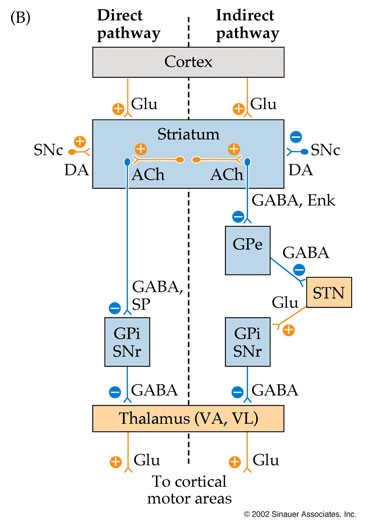
- Joined
- Dec 17, 2010
- Messages
- 263
- Reaction score
- 1
ASK AND ANSWER TOUGH QUESTIONS RELATED TO STEP 1.
Starting with me:
physiologic chloride shift - When CO2 diffuses into a RBC, it quickly converts with H2O to H+ and HCO3- so that CO2 will continue to passively diffuse into the RBC. The HCO3- is then excreted into the plasma by a Cl-/HCO3- exchanger. When the RBC enters the pulmonary capillaries, the process reverses. HCO3- is taken up by exchange for a Cl-. It combines with H+ to creates CO2 +H2O. The CO2 then diffuses out of the RBC and ultimately into the alveoli. This process allows for maximal CO2 excretion by a RBC.
Starting with me:
physiologic chloride shift - When CO2 diffuses into a RBC, it quickly converts with H2O to H+ and HCO3- so that CO2 will continue to passively diffuse into the RBC. The HCO3- is then excreted into the plasma by a Cl-/HCO3- exchanger. When the RBC enters the pulmonary capillaries, the process reverses. HCO3- is taken up by exchange for a Cl-. It combines with H+ to creates CO2 +H2O. The CO2 then diffuses out of the RBC and ultimately into the alveoli. This process allows for maximal CO2 excretion by a RBC.
Last edited: