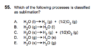- Joined
- Jul 6, 2014
- Messages
- 43
- Reaction score
- 12
I chose C, but the answer key says E is correct.
I assumed H2O(s) --> H2(g) + O2(g). I understand there is a difference between water vapor [H2O(g)] and hydrogen/oxygen gas, but wouldn't both C and E technically be considered "sublimation" reactions? (s-->g)
I assumed H2O(s) --> H2(g) + O2(g). I understand there is a difference between water vapor [H2O(g)] and hydrogen/oxygen gas, but wouldn't both C and E technically be considered "sublimation" reactions? (s-->g)