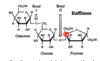
How could the glycosidic bond between fructose and glucose be considered a beta 2--> 1 configuration?
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
TBR Alpha vs Beta anomer
- Thread starter hibni
- Start date
- Joined
- Mar 6, 2013
- Messages
- 135
- Reaction score
- 38
This confused me at first too.
In an anomeric sugar, the way to determine if the anomeric carbon is alpha or beta is NOT simply looking at if it's pointing up or down. No, the technical definition is that the molecule is alpha if the anomeric carbon is TRANS to the terminal carbon.
In D-glucose, when the molecule is oriented so that you're looking at the anomeric carbon on the right, the terminal carbon is pointing UP from the ring. By terminal carbon, I am talking about the the CH2OH (carbon 6) group coming off of carbon 5. If the terminal carbon is pointing UP, than the alpha anomer would have the anomeric OH group pointing DOWN. If it is the beta anomer than the anomeric OH group will point up.
In fructose by itself, we usually look at the molecule with the anomeric carbon on the right side. In this configuration of D-fructose, we see the terminal carbon (the carbon #6 CH2OH group) pointing UP. So just like glucose, we draw the alpha anomer with the anomeric OH group pointing down.
In sucrose, we have a very unique alpha linkage that is different from the alpha linkages we see in starch. In starch, we join two glucoses together in an a1-4 linkage, and all the glucose molecules are oriented in the same direction that we're used to seeing them in.
However, in sucrose, we have a 1-2 linkage, where the anomeric OH of glucose is linked to the anomeric OH of fructose. This is a bit weird. This means that fructose is actually pancake flipped about its vertical axis from how we're used to looking at it. This flippage means that that terminal CH2OH group is now pointing down. What we see in sucrose is that fructose's anomeric OH group is ALSO pointing down, meaning that it is CIS to the fructose terminal carbon, which means that it is actually the beta anomer.
However, we don't really call this a beta glycosidic linkage. The long science name for sucrose is:
O-a-D-glucopyranosyl-(1-->2)-b-D-fructofuranoside
This name tells us that the alpha anomer of glucose is linked to the beta anomer of fructose. What this means is that we can't really call this an alpha or a beta linkage. It's an alpha-beta 1-2 linkage.
There's one other important distinction of sucrose from maltose and starch. In a molecule of maltose (alpha linkage between two D-glucoses), there is a free anomeric OH on that second glucose on the right. It's not bound to anything. An anomeric OH group adjacent to an ether like the ones we see in saccharides is actually called a hemiacetal group. Hemiacetal groups can be spontaneously oxidized into carbonyls. So because D-glucose (and other saccharides) have that free open hemiacetal OH group, it will spontaneously and continuously reform into its straight chain aldose form (ketose for fructose) and back again into an alpha or a beta anomer (there is actually a slight preference for the beta anomer because in the beta orientation the OH group is located on the equatorial plane in the chair conformation). Because the sugar gets oxidized, that makes it a reducing agent. We call sugars that have such a hemiacetal group (a free anomeric OH) reducing sugars.
However, in the a-b 1-2 linkage between two anomeric OH groups like the kind we see in sucrose, there IS NO free anomeric OH group. There is no hemiacetal. Actually, any glycosidic linkage can be referred to as an acetal group. Acetal groups don't have a free OH like hemiacetals do, so they can not spontaneously reform into their straight chains. We call these types of sugars non-reducing sugars. That is why the end of the name for sucrose is furanoside, instead of furanosyl. Non-reducing sugars have an -ide suffix instead of an -yl suffix.
Hope that clears things up a bit
In an anomeric sugar, the way to determine if the anomeric carbon is alpha or beta is NOT simply looking at if it's pointing up or down. No, the technical definition is that the molecule is alpha if the anomeric carbon is TRANS to the terminal carbon.
In D-glucose, when the molecule is oriented so that you're looking at the anomeric carbon on the right, the terminal carbon is pointing UP from the ring. By terminal carbon, I am talking about the the CH2OH (carbon 6) group coming off of carbon 5. If the terminal carbon is pointing UP, than the alpha anomer would have the anomeric OH group pointing DOWN. If it is the beta anomer than the anomeric OH group will point up.
In fructose by itself, we usually look at the molecule with the anomeric carbon on the right side. In this configuration of D-fructose, we see the terminal carbon (the carbon #6 CH2OH group) pointing UP. So just like glucose, we draw the alpha anomer with the anomeric OH group pointing down.
In sucrose, we have a very unique alpha linkage that is different from the alpha linkages we see in starch. In starch, we join two glucoses together in an a1-4 linkage, and all the glucose molecules are oriented in the same direction that we're used to seeing them in.
However, in sucrose, we have a 1-2 linkage, where the anomeric OH of glucose is linked to the anomeric OH of fructose. This is a bit weird. This means that fructose is actually pancake flipped about its vertical axis from how we're used to looking at it. This flippage means that that terminal CH2OH group is now pointing down. What we see in sucrose is that fructose's anomeric OH group is ALSO pointing down, meaning that it is CIS to the fructose terminal carbon, which means that it is actually the beta anomer.
However, we don't really call this a beta glycosidic linkage. The long science name for sucrose is:
O-a-D-glucopyranosyl-(1-->2)-b-D-fructofuranoside
This name tells us that the alpha anomer of glucose is linked to the beta anomer of fructose. What this means is that we can't really call this an alpha or a beta linkage. It's an alpha-beta 1-2 linkage.
There's one other important distinction of sucrose from maltose and starch. In a molecule of maltose (alpha linkage between two D-glucoses), there is a free anomeric OH on that second glucose on the right. It's not bound to anything. An anomeric OH group adjacent to an ether like the ones we see in saccharides is actually called a hemiacetal group. Hemiacetal groups can be spontaneously oxidized into carbonyls. So because D-glucose (and other saccharides) have that free open hemiacetal OH group, it will spontaneously and continuously reform into its straight chain aldose form (ketose for fructose) and back again into an alpha or a beta anomer (there is actually a slight preference for the beta anomer because in the beta orientation the OH group is located on the equatorial plane in the chair conformation). Because the sugar gets oxidized, that makes it a reducing agent. We call sugars that have such a hemiacetal group (a free anomeric OH) reducing sugars.
However, in the a-b 1-2 linkage between two anomeric OH groups like the kind we see in sucrose, there IS NO free anomeric OH group. There is no hemiacetal. Actually, any glycosidic linkage can be referred to as an acetal group. Acetal groups don't have a free OH like hemiacetals do, so they can not spontaneously reform into their straight chains. We call these types of sugars non-reducing sugars. That is why the end of the name for sucrose is furanoside, instead of furanosyl. Non-reducing sugars have an -ide suffix instead of an -yl suffix.
Hope that clears things up a bit