- Joined
- Aug 17, 2014
- Messages
- 64
- Reaction score
- 11
Hey,
I came across a set of questions TBR physics page 96 passage 3 that dealt with measuring the blood pressure by inserting cannula into the artery. The passage stated that average gauge pressure of an artery was 10^4 Pa. One question required the understanding of whether the atmosphere or the artery pressure was greater. I thought that the atmospheric pressure was a lot greater than the gauge pressure since atm pressure = 101.3 kPa. However the answer stated that "Since the average gauge pressure in the artery is greater than zero, the average pressure in the artery is larger than the atmospheric pressure". I know that P total = P gauge + P atm. In this case was I supposed to use the Ptotal (instead of just P gaguge) and compare it with atmospheric pressure? When I think about it conceptually it makes sense, when you get a cut, blood leaks out due to the difference in pressure.
Generally speaking when do I add P atm to P gague?
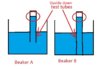
In that drawing that I attached, would I include P atm to calculate the total pressures of fluid in test tube A and test tube B, even though they're not open to the atmosphere directly?
I came across a set of questions TBR physics page 96 passage 3 that dealt with measuring the blood pressure by inserting cannula into the artery. The passage stated that average gauge pressure of an artery was 10^4 Pa. One question required the understanding of whether the atmosphere or the artery pressure was greater. I thought that the atmospheric pressure was a lot greater than the gauge pressure since atm pressure = 101.3 kPa. However the answer stated that "Since the average gauge pressure in the artery is greater than zero, the average pressure in the artery is larger than the atmospheric pressure". I know that P total = P gauge + P atm. In this case was I supposed to use the Ptotal (instead of just P gaguge) and compare it with atmospheric pressure? When I think about it conceptually it makes sense, when you get a cut, blood leaks out due to the difference in pressure.
Generally speaking when do I add P atm to P gague?
In that drawing that I attached, would I include P atm to calculate the total pressures of fluid in test tube A and test tube B, even though they're not open to the atmosphere directly?
Attachments
Last edited: