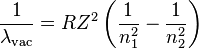- Joined
- Jan 30, 2009
- Messages
- 4,218
- Reaction score
- 13
In TBR Chem Passage XI, they present a data table that indicates that as you go down a column in the periodic table, the energy required to place the outermost electron in an excited state increases.
Is this data scientifically incorrect or is there something I'm missing? Further distance from the nucleus, easier to excite is what makes sense in my mind.
Is this data scientifically incorrect or is there something I'm missing? Further distance from the nucleus, easier to excite is what makes sense in my mind.