TBR GChem II pg 296 #94
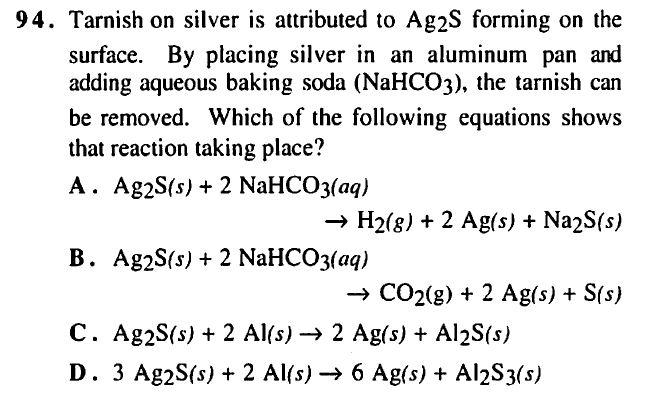
Answer: D
I am having difficulties understanding why B is incorrect. TBR says "If choice B were true, then tarnish could never occur, because the reduction of silver would be carried out by the sulfide anion it binds."
Answer: D
I am having difficulties understanding why B is incorrect. TBR says "If choice B were true, then tarnish could never occur, because the reduction of silver would be carried out by the sulfide anion it binds."
